Abstract
Purpose
Advanced prostate cancer does not respond to traditional androgen deprivation therapy (ADT) at some point in the treatment. The development of new hormonal agents demonstrated clear efficacy and changed the treatment scenario.
Objectives
To evaluate the use of new antiandrogens alone versus their use in combination with maintenance ADT in patients with advanced castration-resistant prostate cancer.
Methods
A literature systematic review of randomized clinical trials, cohorts, and real-life studies including patients who received the new antiandrogens with or without ADT due to histologic confirmed advanced castration-resistant prostate adenocarcinoma was carried out.
Results
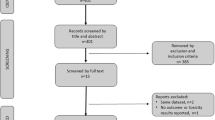
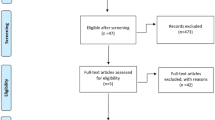
2181 articles were identified and three studies were included with a total of 246 patients. Two studies were randomized clinical trials, and the third was a retrospective study, which showed similar results for both arms, in relation to PSA response, radiological progression-free survival, and testosterone levels, in addition to cost analysis with savings avoided in the ADT maintenance-free arm. Despite the positive data, it is still not possible to categorically state whether there is a statistical benefit in suspending the ADT during the use of new antiandrogens, due to the heterogeneity of the studies.
Conclusion
The literature is limited on the issue. Available data are still immature with no clear benefit of the use of newer antiandrogens alone in the setting of advanced castration-resistant prostate cancer.

Similar content being viewed by others
References
American Cancer Association (2020). Prostate Cancer. [Internet]. Available from: http://www.cancer.org
National Cancer Institute Surveillance. Epidemiology, and results (SEER) Program. CancerStatFactsProstateCancer. Available from: https://seer.cancer.gov/statfacts/html/prost.html
Lowrance WT, Murad MH, Oh WK, Jarrard DF, Resnick MJCM (2018) Castration-resistant prostate cancer: AUA guideline amendment 2018. J Urol 200(6):1264–1272
NCCN clinical practice guideline in oncology (2022). Protate Cancer. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459
Merseburger AS, Hammerer P, Rozet F, Roumeguère T, Caffo O, da Silva FC et al (2015) Androgen deprivation therapy in castrate-resistant prostate cancer: how important is GnRH agonist backbone therapy? World J Urol 33(8):1079–1085
Reis LO, Dal Col LSB, Sadi MV (2021) National consensus on non-metastatic castration-resistant prostate cancer: more than just a snapshot. Int Braz J Urol 47(2):374–377
Dal Col LSB, Andrade DL, Gon LM, Capibaribe DM, Amaro MP, Truzzi NCC, Malkomes BR, Reis LO (2022) Perception of castration value over cost in the metastatic prostate cancer scenario: a contemporary pharmacoeconomic perspective. Int Braz J Urol 48(1):175–179
Reis LO (2012) Variations of serum testosterone levels in prostate cancer patients under LH-releasing hormone therapy: an open question. Endocr Relat Cancer 19(3):R93–R98
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100
Gautam GJ, Engle J (2019) Suppression of testosterone production using abiraterone acetate (AA) with or without androgen deprivation therapy (ADT) in metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol 37(15_suppl):5049–5049
Ohlmann C, Roger Z, Rüssel C, Hellmis E, Suttmann H, Janssen M, Hübner A, Dahm J, Gleißner J, Scheffler M, Feyerabend S, Telle J, Klier J (2018) Abiraterone acetate plus prednisone and LHRH therapy versus abiraterone acetate plus prednisone while sparing LHRH therapy in patients with progressive, metastatic and chemotherapy-naïve, castration-resistant prostate cancer: results from the SPARE-trial (NCT02077634). Ann Oncol 29(suppl_8):viii271–viii302
Maluf FC, Fay AP, Souza VC, Schutz FAB, Smaletz O, Herchenhorn D, Fabricio V, Gidekel R, Cronemberger E, Luz M, Martins SPS, Muniz DQB, Franke FA, Peixoto F, Carcano FM, Gomes AJ, Cruz F, Gomes R, Filho PRSN, Werutsky G (2020) Phase II randomized study of abiraterone acetate plus prednisone (AAP) added to ADT versus apalutamide alone (APA) versus AAP+APA in patients with advanced prostate cancer with noncastrate testosterone levels: (LACOG 0415). J Clin Oncol 38(15_suppl):5505
Saad ED, Buyse M (2016) Statistical controversies in clinical research: end points other than overall survival are vital for regulatory approval of anticancer agents. Ann Oncol 27(3):373–378
dos Santos LF, de Morais AE, Furtado AB, Pinto BNSL, da Martins SKR, Alves EB et al (2019) Farmacovigilância de polifarmácia e reações adversas medicamentosas em idosos hospitalizados em hospital universitário de Manaus, Amazonas. Vigilância Sanitária em Debate 7(4):41
McLean AJ, Le Couteur DG (2004) Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56(2):163–184
Del Paggio JC, Sullivan R, Schrag D, Hopman WM, Azariah B, Pramesh CS, Tannock IFBC (2017) Delivery of meaningful cancer care: a retrospective cohort study assessing cost and benefit with the ASCO and ESMO frameworks. Lancet Oncol 18(7):887–894
Caffo O, Palesandro E, Nole F, Gasparro D, Mucciarini C, Aieta M, Zagonel V, Iacovelli R, De Giorgi U, Rossetti S, Fratino L, Sacco C, Nicodemo M, Giordano M, Sartori D, Scapoli D, Verri E, Kinspergher S, Pappagallo GL, Aglietta M (2019) A multicentric phase II randomized trial of docetaxel (D) plus enzalutamide (E) versus docetaxel (D) as first-line chemotherapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): CHEIRON study. J Clin Oncol 37(7_suppl):148–148
Fizazi K, Maldonado X, Foulon S, Roubaud G, McDermott RS, Flechon A, Tombal BF, Supiot S, Berthold DR, Ronchin P, Kacso G, Gravis G, Calabro F, Berdah JF, Hasbini A, Silva M, Thiery-Vuillemin A, Rieger I, Tanguy ML, Bossi A (2021) A phase 3 trial with a 2x2 factorial design of abiraterone acetate plus prednisone and/or local radiotherapy in men with de novo metastatic castration-sensitive prostate cancer (mCSPC): First results of PEACE-1. J Clin Oncol 39(15_suppl):5000
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Author CMS, HGP, and LOR declare that they have no conflict of interest.
Research involved with human participants
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salgado, C.M., Garcia-Perdomo, H.A. & Reis, L.O. Evaluation of maintenance of the common androgen deprivation therapy with the new antiandrogen therapy in patients with castration-resistant prostate cancer: a systematic review. Int Urol Nephrol 54, 1187–1192 (2022). https://doi.org/10.1007/s11255-022-03201-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03201-9