Abstract
Purpose
Cisplatin has been widely accepted as an effective chemotherapy drug with various side effects, including nephrotoxicity. The mechanisms of cisplatin-induced acute kidney injury (AKI) are complex, and there are limited renoprotective approaches. Leonurine is the main active compound of a Chinese herb and has recently been reported to have a protective effect on the kidneys. This study aimed to verify the renoprotective effect of leonurine in attenuating cisplatin-induced AKI and explore the potential associated mechanisms.
Methods
C57BL/6 mice were divided into four groups (Sham, Cisplatin, Leonurine, and Cisplatin + Leonurine). Mice in the leonurine-treated groups were pretreated with a daily intraperitoneal injection of 25 mg/kg leonurine. AKI was induced by injecting cisplatin once intraperitoneally at 20 mg/kg body weight. Mice were killed on day 5. Kidney injury was assessed using a serum biochemical and histological assay. Apoptosis was evaluated using a terminal deoxyribonucleotide transferase-mediated dUTP nick-end labeling (TUNEL) staining assay and Western blot. Antioxidant enzymes were detected using commercial kits. The improvement in inflammasome activation, mitochondrial dysfunction, and endoplasmic reticulum stress (ERS) were assessed by polymerase chain reaction (PCR) and Western blot, respectively.
Results
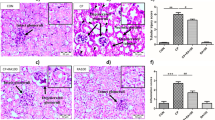
Leonurine treatment improved kidney function by preventing renal tubular injury and apoptosis. Expression of nucleotide-binding leucine-rich repeat and pyrin domain containing protein 3 (NLRP3) inflammasome components and inflammatory cytokines, mitochondrial dysfunction, and ERS were all alleviated by leonurine.
Conclusion
The results indicate that leonurine plays a protective role in cisplatin-induced AKI and may represent an effective multi-targeted intervention strategy.







Similar content being viewed by others
References
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4(4):307–320
Kociba RJ, Sleight SD (1971) Acute toxicologic and pathologic effects of cis-diamminedichloroplatinum (NSC-119875) in the male rat. Cancer Chemother Rep 55(1):1–8
Goldstein RS, Mayor GH (1983) Minireview. The nephrotoxicity of cisplatin. Life Sci 32(7):685–690
Madias NE, Harrington JT (1978) Platinum nephrotoxicity. Am J Med 65(2):307–314
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73(9):994–1007
Bajwa A et al (2015) Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol 26(4):908–925
Guo Y et al (2018) MicroRNA-709 mediates acute tubular injury through effects on mitochondrial function. J Am Soc Nephrol 29(2):449–461
Un H et al (2020) Phloretin and phloridzin guard against cisplatin-induced nephrotoxicity in mice through inhibiting oxidative stress and inflammation. Life Sci 266:118869
Swanson KV et al (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19(8):477–489
Xu Y et al (2019) Prohibitin 2-mediated mitophagy attenuates renal tubular epithelial cells injury by regulating mitochondrial dysfunction and NLRP3 inflammasome activation. Am J Physiol Renal Physiol 316(2):F396–F407
Bi X et al (2018) MnTBAP treatment ameliorates aldosterone-induced renal injury by regulating mitochondrial dysfunction and NLRP3 inflammasome signalling. Am J Transl Res 10(11):3504–3513
Jiang S et al (2020) Vitamin D/VDR attenuate cisplatin-induced AKI by down-regulating NLRP3/Caspase-1/GSDMD pyroptosis pathway. J Steroid Biochem Mol Biol 206:105789
Yang SK et al (2020) Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed Pharmacother 130:110521
Li S et al (2019) NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp Cell Res 383(1):111488
Gao H et al (2020) Omeprazole attenuates cisplatin-induced kidney injury through suppression of the TLR4/NF-kappaB/NLRP3 signaling pathway. Toxicology 440:152487
Cybulsky AV (2017) Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13(11):681–696
Foufelle F, Fromenty B (2016) Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect 4(1):e00211
Liu H, Baliga R (2005) Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16(7):1985–1992
Peyrou M et al (2007) Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci 99(1):346–353
Zhang Y et al (2012) SCM-198 attenuates early atherosclerotic lesions in hypercholesterolemic rabbits via modulation of the inflammatory and oxidative stress pathways. Atherosclerosis 224(1):43–50
Xu D et al (2014) Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia 97:148–155
Cheng H et al (2015) Leonurine ameliorates kidney fibrosis via suppressing TGF-beta and NF-kappaB signaling pathway in UUO mice. Int Immunopharmacol 25(2):406–415
Liu X et al (2018) Leonurine ameliorates adriamycin-induced podocyte injury via suppression of oxidative stress. Free Radic Res 52(9):952–960
Liu Y et al (2018) Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicol Appl Pharmacol 351:1–11
Lieberthal W et al (1996) Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol 270(4 Pt 2):F700–F708
Ramesh G, Reeves WB (2003) TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285(4):F610–F618
Cilenti L et al (2005) Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am J Physiol Renal Physiol 288(2):F371–F379
Park MS et al (2002) Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol 13(4):858–865
Jiang M et al (2006) Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25(29):4056–4066
Wei Q et al (2007) The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72(1):53–62
Yin X et al (2007) Induction of renal endonuclease G by cisplatin is reduced in DNase I-deficient mice. J Am Soc Nephrol 18(9):2544–2553
Jiang M et al (2004) Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287(6):F1140–F1147
Kaushal GP et al (2001) Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int 60(5):1726–1736
Nagothu KK et al (2005) Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int 68(6):2680–2693
Wang J et al (2004) Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279(19):19948–19954
Baliga R et al (1997) Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis 29(3):465–477
Siddik ZH (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22(47):7265–7279
Liu H, Baliga R (2003) Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int 63(5):1687–1696
Kruidering M et al (1997) Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther 280(2):638–649
Li S et al (2004) PPAR alpha ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol 286(3):F572–F580
Portilla D et al (2002) Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int 62(4):1208–1218
Brooks C et al (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119(5):1275–1285
Larsson NG et al (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18(3):231–236
Abais JM et al (2015) Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 22(13):1111–1129
Zhang Y et al (2014) P2X7 receptor blockade protects against cisplatin-induced nephrotoxicity in mice by decreasing the activities of inflammasome components, oxidative stress and caspase-3. Toxicol Appl Pharmacol 281(1):1–10
Liu M et al (2006) A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol 17(3):765–774
Ramesh G, Reeves WB (2002) TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110(6):835–842
Faubel S et al (2007) Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322(1):8–15
Periyasamy-Thandavan S et al (2008) Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74(5):631–640
Yang C et al (2008) Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol 294(4):F777–F787
Yu X et al (2020) Nuclear receptor PXR targets AKR1B7 to protect mitochondrial metabolism and renal function in AKI. Sci Transl Med 12:543
Zhong Z et al (2016) NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164(5):896–910
Han J et al (2016) Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice. Autophagy 12(12):2326–2343
Qu X et al (2018) Autophagy inhibition-enhanced assembly of the NLRP3 inflammasome is associated with cisplatin-induced acute injury to the liver and kidneys in rats. J Biochem Mol Toxicol 33:e22208
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Wang M, Kaufman RJ (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529(7586):326–335
Huang Z et al (2020) Activation of GPR120 by TUG891 ameliorated cisplatin-induced acute kidney injury via repressing ER stress and apoptosis. Biomed Pharmacother 126:110056
Funding
This work was supported by grants from the National Natural Science Foundation of China (81970609); “The Belt and Road international cooperation project” (18410741500) and “Interdisciplinary program of Shanghai Jiao Tong University” (YG2017MS07) and “Science and Technology Innovation Fund of Shanghai Ninth People’s Hospital” (CK2019010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval
All the animal experiments were approved by the Animal Care Committee at Shanghai Jiao Tong University and carried out in accordance with institutional guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., Sun, Q., Tong, Y. et al. Leonurine attenuates cisplatin nephrotoxicity by suppressing the NLRP3 inflammasome, mitochondrial dysfunction, and endoplasmic reticulum stress. Int Urol Nephrol 54, 2275–2284 (2022). https://doi.org/10.1007/s11255-021-03093-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-03093-1