Abstract
Purpose
To assess the efficacy of prophylaxis for urinary tract infections (UTI) in a two-year follow-up in women with StroVac compared to a therapy with Nitrofurantoin over three months.
Materials and methods
All patients with documented recurrent urinary tract infections (rUTI) were offered vaccination with StroVac or therapy with three months Nitrofurantoin 100 mg once daily for three months at patient’s choice. Only patients with a follow-up of at least 24 months were included. All episodes with signs of UTI were documented and urine culture was performed. Success was defined as one or none UTI per 12 months, documented by urine culture. StroVac booster injection was offered 12 months after primary vaccination at patient’s choice.
Results
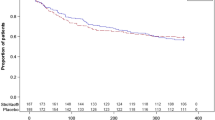
173 patients were included in this study, 124 in the StroVac group, 49 chose Nitrofuratoin. In the first 12 months, 86.8% of patients in the StroVac group and 91.8% in Nitrofurantoin group were successful (p = 0.22). Side effects were noted in 2.3% in the StroVac group causing discontinuation of therapy, whereas in the Nitrofurantoin group 18.4% stopped medication premature, mostly due to mild diarrhoea. In the second year 79.3% of patients in the StroVac group were still successful, most of them had undergone booster injection. In contrast, in the Nitrofurantoin group only 59.2% of patients were still successful (p = 0.03).
Conclusion
StroVac is an effective and lasting non-antibiotic prophylaxis for rUTI, easy to administer with low rates of adverse events and should be offered to patients with rUTI.
Similar content being viewed by others
References
Foxman B, Brown P (2003) Epidemiology of urinary tract infections: transmission and risk factors incidence and costs. Infect Dis Clin North Am 17:227–241
Ikäheimo R, Siitonen A, Heiskanen T, Kärkkäinen U (1996) Recurrence of urinary tract infection in a primary case setting: analysis of a 1-year-folow-up in 179 women. Clin Infect Dis 22:91–99
Sivick KE, Mobley KL (2010) Waging war against uropathogenic escherichia coli: winning back the urinary tract. Infect Immun 78(2):568–585
Gupta K, Sahm DF, Mayfield D, Stamm WE (2001) Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis 33(1):89–94
Nickel JC (2005) Practical management of recurrent urinary tract infections in premenopausal women. Rev Urol 7:11–17
Kranjec B, Papes D, Altarac S (2014) D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clnical trial. World J Urol 32:79–84
Albert X, Huertas I, Pereiro II, Sanfelix J, Gosalbes V, Perrote C (2004) Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 3:CD001209
Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L, Marchese A (2009) The ARESC study: an international survey on the antimicrobial resistence of pathogens involved in uncomplicates urinary tract infections. Int J Antimicrob Agents 34:407–413
Zhanel GG, Hisanaga TL, Laing NM, DeCorby MR, Nichol KA, Palatnik LP, Johnson J, Noreddin A, Harding GK, Nicolle LE, Hoban DJ (2005) Antibiotic resistance in outpatient urinary isolates: final results from the North American urinary tract infection collaborative alliance (NAUTICA). Int J Antimicrob Agents 26:380–388
Gupta K, Stamm WE (1999) Pathogenesis and management of recurrent urinary tract infections in women. World Jour Urol 17:415–420
Kranz J, Schmidt S, Lebert C, Schneidewind L, Mandraka L, Kunze M, Helbig S, Vahlensieck W, Naber K, Schmiemann G, Wagenlehner FM (2018) The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients. Part II: therapy and prevention. Urol Int 100(3):271–278
EAU Guideline on urological infections, https://uroweb.org/guideline/urological-infections/#3_5. Accessed 14 May 2021
Aziminia N, Hadjipvlou M, Philippou Y, Pandian SS, Malde S, Hammadeh MY (2019) Vaccines for the prevention of recurrent urinary tract infections: a systematic review. BJUI 123(5):753–768
Grischke EM, Rüttgers H (1987) Treatment of bacterial infections of the female urinary tract by immunization of the patients. Urol Int 42(5):338–341
Naber GK, Cho YH, Matsumoto T, Schaeffer AJ (2009) Immunoactive prophylaxis of recurrent urinary tract infections: a meta-analysis. Int J Antimicrob Agents 33(2):111–119
Beerepoot MA, Geerlings SE (2016) Non-antibiotic prophylaxis for urinary tract infections. Pathogens 5(2):36
The German Urinary Tract Infection Study Group, Tammen H (1990) Immunotherapy with Uro-Vaxom in recurrent urinary tract infection. Br J Urol 65:6–9
Magasi P, Panovics J, Illes A, Nagy M (1994) Uro-Vaxom and the management of recurrent urinary tract infection in adults: a randomized multicenter double-blind trial. Eur Urol 26:137–140
Nayir A, Emre S, Sirin A, Bulut A, Alpay H, Tanman F (1995) The effects of vaccination with inactivated uropathogenic bacteria in recurrent urinary tract infections of children. Vaccine 13(11):987–990
Hopkins WJ, Elkahwaji J, Beierle LM, Leverson GE, Uehling DT (2007) Vaginal mucosal vaccine for recurrent urinary tract infections in women: results of a phase 2 clinical trial. J Urol 177:1349–1353
Prospective multicentre randomized double-blind placebo-controlled parallel group study on the efficacy and tolerability of StroVac® in patients with recurrent symptomatic bacterial urinary tract infections, https://portal.dimdi.de/data/ctr/O-2602490-3-0-89591F-20160421135205.pdf. Accessed 14 May 2021
Wagenlehner FM, Ballarini S, Pilatz A, Weidner W, Lehr L, Naber KG (2015) A randomized, double-blind, parallel-group, multicenter clinical study of escherichia coli-lyophilized lysate for the prophylaxis of recurrent uncomplicated urinary tract infections. Urol Int 95(2):167–176
Ivanov D, Abramov-Sommariva D, Moritz K, Eskötter H, Kostinenko T, Martynyuk L, Kolesnik N, Naber K (2015) An open label, non-controlled, multicentre, interventional trial to investigate the safety and efficacy of Canephron N in the management of uncomplicated urinary tract infections (uUTIs). Clin Phytosci 1:7
Yang B, Foley S (2019) Urinary tract infection vaccines—the ‘burning’ issue. BJU Int 123(5):743–744
Lenk S, Dorsch B (2009) Vaccinations in recurrent urinary tract infections–an observation study relating to health economy. Aktuelle Urol 40(6):360–365
Beerepoot MA, Geerlings SE, van Haarst EP, Mensing van Charante N, ter Riet G (2013) Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol 190(6):1981–1989
Bauer HW, Rahlfs VW, Lauener PA, Bleßmann GS (2002) Prevention of recurrent urinary tract infections with immuno-active E.coli fractions: a meta-analysis of five placebo-controlled double-blind studies. Int J Antimic agents 19:451–456
Funding
This research received no specific grant from any funding agency in the public, commercial or non-profit sector.
Author information
Authors and Affiliations
Contributions
SN designed the study, SN, AS and AP and collected the data, SN wrote the manuscript with input regarding statistics from BG and LS, AN and SN edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethics approval
Ethics approval was obtained by the internal ethics board. All patients were treated with standard of care medication regarding the participating urological practice. Informed consent was obtained by all patients regarding the collected information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nestler, S., Grüne, B., Schilchegger, L. et al. Efficacy of vaccination with StroVac for recurrent urinary tract infections in women: a comparative single-centre study. Int Urol Nephrol 53, 2267–2272 (2021). https://doi.org/10.1007/s11255-021-02987-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-02987-4