Abstract
The benefits from cardiovascular implantable electronic devices (CIED) implantation in hemodialysis (HD) patients are still far to be thoroughly defined, especially on primary prevention. In addition, CIED placement is not a risk-free procedure, because it could be followed by a not negligible burden of complications that could compromise the health and the vascular access of HD patients. In fact, the arteriovenous fistula (AVF) dysfunction following CIED implantation is usually due to a hemodynamically significant alteration of blood flow. This condition could lead to a potential decrease of dialysis efficacy and a raised risk of thrombosis of both the central vein and the efferent vein of the AVF.
The pathological pathway that leads to AVF dysfunction after CIED implantation may involve the irritating actions of the CIED and their leads to the vascular wall in HD patients that are more prone to show previous vascular diseases.
The aim of this review is to focus the physiopathology of the CIED-induced AVF dysfunction, the current treatment strategies and the novel perspectives that could be taken into consideration and offered to the HD population to preserve both their AVF and their quality of life.


Similar content being viewed by others
References
Vascular Access 2006 Work Group (2006) Clinical practice guidelines for vascular access. Am J Kidney Dis 48(Suppl 1):S176–S247
Konner K, Nonnast-Daniel B, Ritz E (2003) The arteriovenous fistula. J Am Soc Nephrol 14:1669–1680
Lindfors O, Paldanius R (1976) Experience with arteriovenous fistulas CIMINO in chronic hemodialysis. A report on forty-five patients. Scand J Urol Nephrol 10(1):80–83 (PubMed: 1273536)
Paruk S, Koenig M, Levitt S, Hardy MA (1976) Arteriovenous fistulas for hemodialysis in 100 consecutive patients. Am J Surg 131(5):552–555 (PubMed: 818910)
Al-Jaishi AA, Oliver MJ, Thomas SM et al (2014) Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 63(3):464–478. https://doi.org/10.1053/j.ajkd.2013.08.023 (Epub 2013 Oct 30. Review)
Irish A, Dogra G, Mori T et al (2009) Preventing AVF thrombosis: the rationale and design of the Omega-3 fatty acids (Fish Oils) and Aspirin in Vascular access OUtcomes in REnal Disease (FAVOURED) study. BMC Nephrol 21(10):1. https://doi.org/10.1186/1471-2369-10-1
Di Lullo L, Rivera R, Barbera V et al (2016) Sudden cardiac death and chronic kidney disease: from pathophysiology to treatment strategies. Int J Cardiol 217:16–27. https://doi.org/10.1016/j.ijcard.2016.04.170 (Epub 2016 May 3. Review)
El-Chami MF, Matar L, Smith P (2017) Long-term survival of implantable cardioverter defibrillator recipients with end-stage renal disease. J Arrhythm 33:459–462
Wan C, Herzog CA, Zareba W, Szymkiewicz SJ (2014) Sudden cardiac arrest in hemodialysis patients with wearable cardioverter defibrillator. Ann Noninvasive Electrocardiol 19:247–257
Russo AM, Stainback RF, Bailey SR et al (2013) ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm Off J Heart Rhythm Soc 10(4):e11-58
USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 201
Saad TF, Ahmed W, Davis K (2015) Cardiovascular implantable electronic devices in hemodialysis patients: prevalence and implications for arteriovenous hemodialysis access interventions. Semin Dial 28:94–100
Aggarwal A, Wang Y, Rumsfeld JS, Curtis JP, Heidenreich PA, National Cardiovascular Data Registry (2009) Clinical characteristics and in-hospital outcome of patients with end-stage renal disease on dialysis referred for implantable cardioverter-defibrillator implantation. Heart Rhythm 6(11):1565–1571. https://doi.org/10.1016/j.hrthm.2009.08.006
Dasgupta A, Montalvo J, Medendorp S et al (2007) Increased complication rates of cardiac rhythm management devices in ESRD patients. Am J Kidney Dis 49(5):656–663
Asif A, Salman L, Lopera G, Haqqie SS, Carrillo R (2012) Transvenous cardiac implantable electronic devices and hemodialysis catheters: recommendations to curtail a potentially lethal combination. Semin Dial 25:582–586
Bloom H, Heeke B, Leon A et al (2006) Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol 29:142–145
Tompkins C, McLean R, Cheng A et al (2011) End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 22(10):1099–1104
Brunner MP, Cronin EM, Duarte VE et al (2014) Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 11:799–805
Herzog CA, Li S, Weinhandl ED et al (2005) Survival of dialysis arrest and the impact of implantable cardioverter defibrillators. Kidney Int 68(818–825):95
Haghjoo M, Nikoo MH, Fazelifar AF (2007) Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace 9:328–332
Kusztal M, Nowak K (2018) Cardiac implantable electronic device and vascular access: strategies to overcome problems. J Vasc Access 19(6):521–527. https://doi.org/10.1177/1129729818762981
Teruya TH, Abou-Zamzam AM, Limm W (2003) Symptomatic subclavian vein stenosis and occlusion in hemodialysis patients with transvenous pacemakers. Ann Vasc Surg 17:526–529
Drew DA, Myers KB, Weiner DE (2011) Transvenous cardiac device wires and vascular access in hemodialysis patients. Am J Kidney Dis 58:494–495
Asif A, Salman L, Carrillo RG (2009) Patency rates for angioplasty in the treatment of pacemaker-induced central venous stenosis in hemodialysis patients: results of a multi-center study. Semin Dial 22:671
Surowiec SM, Fegley AJ, Tanski WJ et al (2004) Endovascularmanagement of central venous stenoses in the hemodialysis patient: results of percutaneous therapy. Vasc Endovascular Surg 38(4):349–354
Wilkoff BL, Love CJ, Byrd CL et al (2009) Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 6:1085–1104
Da Costa A, Lelievre H, Pha D, Kirkorian G, Celard M, Chevalier P et al (1998) Role of the preaxillary flora in pacemaker infections. Circulation 97:1791–1795
Hussein AA, Baghdy Y, Wazni OM, Brunner MP, Kabbach G, Shao M et al (2016) Microbiology of cardiac implantable electronic device infections. JACC: Clin Electrophysiol 2:498–505
Le KY, Sohail MR, Friedman PA, Uslan DZ, Cha SS, Hayes DL et al (2011) Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm 8:1678–1685
Lebeaux D, Fernandez-Hidalgo N, Chauhan A, Lee S, Ghigo J-M, Almirante B et al (2014) Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis 14:146–159
Blomstrom-Lundqvist C, Traykov V, Erba PA et al (2020) European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 22(4):515–549. https://doi.org/10.1093/europace/euz246
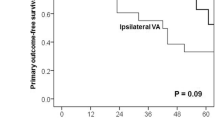
Sgroi MD, McFarland G (2019) Arteriovenous Fistula and graft construction in patients with implantable cardiac devices: does side matter. Ann Vasc Surg 54:66–71. https://doi.org/10.1016/j.avsg.2018.10.003 (Epub 2018 Oct 17.PMID: 30339901)
Fila B (2020) Quality indicators of vascular access procedures for hemodialysis. Int Urol Nephrol. https://doi.org/10.1007/s11255-020-02609-5
Koirala N, Anvari E, McLennan G (2016) Monitoring and surveillance of hemodialysis access. Semin Intervent Radiol 33(1):25–30. https://doi.org/10.1055/s-0036-1572548
D'Onofrio G, Simeoni M, Rizza P et al. (2017) Quality of life, clinical outcome, personality and coping in chronic hemodialysis patients. Ren Fail. (PMID: 27778533)
Sabet R, Naghizadeh MM, Azari S (2012) Quality of sleep in dialysis patients. Iran J Nurs Midwifery Res 17:270–274
Iliescu EA, Yeates KE, Holland DC (2004) Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant 19:95–99
Zenati MA, Bonanomi G, Chin A et al (2003) Left heart pacing lead implantation using subxiphoid videopericardioscopy. J Cardiovasc Electrophysiol 14(9):949–953
DeRose JJ, Ashton RC, Belsley S et al (2003) Robotically assisted left ventricular epicardial lead implantation for biventricular pacing. J Am Coll Cardiol 41:1414–1419
Asif A, Carrillo R, Garisto JD et al (2012) Epicardial cardiac rhythm devices for dialysis patients: minimizing the risk of infection and preserving central veins. Semin Dial 25:88–94
Koman E, Gupta A, Subzposh F et al (2016) Outcomes of subcutaneous implantable cardioverter-defibrillator implantation in patients on hemodialysis. J Interv Card Electrophysiol 45:219–223
Burke MC, Gold MR, Knight BP et al (2015) Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am CollCardiol 65(16):1605–1615
Hess PL, Hellkamp AS, Peterson ED et al (2014) Survival after primary prevention implantable cardioverter-defibrillator placement among patients with chronic kidney disease. Circ Arrhythm Electrophysiol 7(5):793–799. https://doi.org/10.1161/CIRCEP.114.001455 (Epub 2014 Jul 18)
Kondo Y, Ueda M, Kobayashi Y, Schwab JO (2016) New horizon for infection prevention technology and implantable device. J Arrhythm 32(4):297–302
Piro A, Lavalle C, Della Rocca DG et al (2019) Leadless pacing: present and future of cardiac stimulation. G ItalCardiol (Rome) 20(1):32–40. https://doi.org/10.1714/3079.30718
El Charmi M, Clementy N, Garweg C et al (2019) Leadless pacemaker implantation in hemodialysis patients: experience with the micra transcatheter pacemaker. JACC Clin Electrophysiol 5(2):162–170. https://doi.org/10.1016/j.jacep.2018.12.008
Ritter P, Duray GZ, Zhang S, Narasimhan C, Soejima K, Omar R, Laager V, Stromberg K, Williams E, Reynolds D (2015) The rationale and design of the Micra trans-catheter pacing study: safety and efficacy of a novel miniaturized pacemaker. Europace 17:807–813
Kay G, Eby EL, Brown B et al (2018) Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ 21(3):294–300. https://doi.org/10.1080/13696998.2017.1409227 (Epub 2018 Jan 3 PMID: 29171319)
Kolek MJ, Patel NJ, Clair WK (2015) Efficacy of a bio-absorbable antibacterial envelope to prevent cardiac implantable electronic device infections in high-risk subjects. J Cardiovasc Electrophysiol 26:1111–1116
Chatzinikolaou I, Finkel K, Hanna H (2003) Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med 115:352–357
Acknowledgements
All the authors disclose any conflict of interest and any funding for the preparation of this manuscript.
Funding
No funding was requested for the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to prepare the manuscript by evaluating and assessing the current literature on this topic.
Corresponding author
Ethics declarations
Conflict of interest
All authors disclose any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spatola, L., Rivera, R.F. & Mugnai, G. Cardiovascular implantable electronic devices and native arteriovenous fistula in hemodialysis patients: novel perspectives. Int Urol Nephrol 53, 2541–2548 (2021). https://doi.org/10.1007/s11255-021-02830-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-02830-w