Abstract
Purpose
Diabetic nephropathy (DN) is a major complication of diabetic mellitus and usually leads to the end-stage renal disease. Inflammation-induced lipid disorders have been proposed to play an important role in the pathogenesis of DN. S100A16 is a novel adipogenic factor, but has not been investigated in DN. This study aims to explore the role of S100A16 in high glucose (HG)-induced HK-2 cells.
Methods
CCK-8 assay was used to detect cell viability. Cell transfection was performed to knockdown S100A16. Oil red staining was performed to assay lipid accumulation. qRT-PCR and western blotting were conducted to examine corresponding gene expression. Intracellular cholesterol was determined by enzymatic assay. Inflammatory cytokines production was measured using ELISA kits.
Results
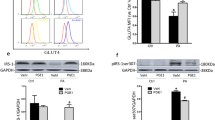
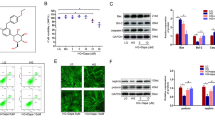
The results exhibited lipid accumulation and upregulation of S100A16 in HG-induced HK-2 cells. S100A16 knockdown significantly reduced lipid droplets and cholesterol, and decreased the production of inflammatory cytokines induced by HG. Besides, S100A16 knockdown decreased the expression of SCAP, SREBP1, SCD1 and SCAP. However, the inhibitory effect in HG-induced HK-2 cells made by S100A16 was reversed by SREBP1 overexpression.
Conclusion
These results suggested that S100A16 knockdown might protect against HG-induced lipid accumulation and inflammation in HK-2 cells through regulating SCAP/SREBP1 signaling.








Similar content being viewed by others
References
Lee JH, Kim D, Oh YS, Jun HS (2019) Lysophosphatidic acid signaling in diabetic nephropathy. Int J Mol Sci. https://doi.org/10.3390/ijms20112850
Kihm L (2016) Hypertension and diabetic nephropathy. Exp Clin Endocrinol Diabetes 124(6):333–334. https://doi.org/10.1055/s-0042-110231
Disease GBD, Injury I, Prevalence C (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7
Selby NM, Taal MW (2020) An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab 22(Suppl 1):3–15. https://doi.org/10.1111/dom.14007
Maezawa Y, Takemoto M, Yokote K (2015) Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig 6(1):3–15. https://doi.org/10.1111/jdi.12255
Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L, Lu J, Zhang XL, Liu BC (2015) Inflammatory stress exacerbates lipid accumulation and podocyte injuries in diabetic nephropathy. Acta Diabetol 52(6):1045–1056. https://doi.org/10.1007/s00592-015-0753-9
Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, Scherzer P, Lewis L, Miyazaki-Anzai S, Levi M (2009) The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol 297(6):F1587-1596. https://doi.org/10.1152/ajprenal.00404.2009
Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U (2014) Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 55(3):561–572. https://doi.org/10.1194/jlr.P040501
Sakamoto A, Hongo M, Saito K, Nagai R, Ishizaka N (2012) Reduction of renal lipid content and proteinuria by a PPAR-gamma agonist in a rat model of angiotensin II-induced hypertension. Eur J Pharmacol 682(1–3):131–136. https://doi.org/10.1016/j.ejphar.2012.02.027
Marenholz I, Heizmann CW (2004) S100A16, a ubiquitously expressed EF-hand protein which is up-regulated in tumors. Biochem Biophys Res Commun 313(2):237–244. https://doi.org/10.1016/j.bbrc.2003.11.115
Liu Y, Zhang R, Xin J, Sun Y, Li J, Wei D, Zhao AZ (2011) Identification of S100A16 as a novel adipogenesis promoting factor in 3T3-L1 cells. Endocrinology 152(3):903–911. https://doi.org/10.1210/en.2010-1059
Li Q, Baines KJ, Gibson PG, Wood LG (2016) Changes in expression of genes regulating airway inflammation following a high-fat mixed meal in asthmatics. Nutrients. https://doi.org/10.3390/nu8010030
Sturchler E, Cox JA, Durussel I, Weibel M, Heizmann CW (2006) S100A16, a novel calcium-binding protein of the EF-hand superfamily. J Biol Chem 281(50):38905–38917. https://doi.org/10.1074/jbc.M605798200
Xu HF, Luo J, Zhao WS, Yang YC, Tian HB, Shi HB, Bionaz M (2016) Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J Dairy Sci 99(1):783–795. https://doi.org/10.3168/jds.2015-9736
Williams KJ, Argus JP, Zhu Y, Wilks MQ, Marbois BN, York AG, Kidani Y, Pourzia AL, Akhavan D, Lisiero DN, Komisopoulou E, Henkin AH, Soto H, Chamberlain BT, Vergnes L, Jung ME, Torres JZ, Liau LM, Christofk HR, Prins RM, Mischel PS, Reue K, Graeber TG, Bensinger SJ (2013) An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res 73(9):2850–2862. https://doi.org/10.1158/0008-5472.CAN-13-0382-T
Liu C, Zhuo H, Ye MY, Huang GX, Fan M, Huang XZ (2020) LncRNA MALAT1 promoted high glucose-induced pyroptosis of renal tubular epithelial cell by sponging miR-30c targeting for NLRP3. Kaohsiung J Med Sci. https://doi.org/10.1002/kjm2.12226
Cao Z, Cooper ME (2011) Pathogenesis of diabetic nephropathy. J Diabetes Investig 2(4):243–247. https://doi.org/10.1111/j.2040-1124.2011.00131.x
Yacoub R, Campbell KN (2015) Inhibition of RAS in diabetic nephropathy. Int J Nephrol Renovasc Dis 8:29–40. https://doi.org/10.2147/IJNRD.S37893
Ferrao FM, Lara LS, Lowe J (2014) Renin-angiotensin system in the kidney: what is new? World J Nephrol 3(3):64–76. https://doi.org/10.5527/wjn.v3.i3.64
Mohamed RH, Abdel-Aziz HR, Abd El Motteleb DM, Abd El-Aziz TA (2013) Effect of RAS inhibition on TGF-beta, renal function and structure in experimentally induced diabetic hypertensive nephropathy rats. Biomed Pharmacother 67(3):209–214. https://doi.org/10.1016/j.biopha.2009.08.002
Lv N, Li C, Liu X, Qi C, Wang Z (2019) miR-34b Alleviates high glucose-induced inflammation and apoptosis in human HK-2 cells via IL-6R/JAK2/STAT3 signaling pathway. Med Sci Monit 25:8142–8151. https://doi.org/10.12659/MSM.917128
Zhang J, Zhao X, Zhu H, Wang J, Ma J, Gu M (2019) Apigenin protects against renal tubular epithelial cell injury and oxidative stress by high glucose via regulation of NF-E2-related factor 2 (Nrf2) pathway. Med Sci Monit 25:5280–5288. https://doi.org/10.12659/MSM.915038
Dominguez B, Pardo BG, Noia M, Millan A, Gomez-Tato A, Martinez P, Leiro J, Lamas J (2013) Microarray analysis of the inflammatory and immune responses in head kidney turbot leucocytes treated with resveratrol. Int Immunopharmacol 15(3):588–596. https://doi.org/10.1016/j.intimp.2013.01.024
Wang X, Sato R, Brown MS, Hua X, Goldstein JL (1994) SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell 77(1):53–62. https://doi.org/10.1016/0092-8674(94)90234-8
Xu M, Jiang F, Li B, Zhang Z (2019) 1Alpha, 25(OH)2 D3 alleviates high glucose-induced lipid accumulation in rat renal tubular epithelial cells by inhibiting SREBPs. J Cell Biochem 120(9):15211–15221. https://doi.org/10.1002/jcb.28786
Sun H, Yuan Y, Sun ZL (2013) Cholesterol contributes to diabetic nephropathy through SCAP-SREBP-2 pathway. Int J Endocrinol 2013:592576. https://doi.org/10.1155/2013/592576
Wang XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, Wang D, Peng Y, Grenz A, Lucia S, Dobrinskikh E, D’Agati VD, Koepsell H, Kopp JB, Rosenberg AZ, Levi M (2017) SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem 292(13):5335–5348. https://doi.org/10.1074/jbc.M117.779520
Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD (2012) The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab 15(2):240–246. https://doi.org/10.1016/j.cmet.2011.12.017
Jiang X, Yu J, Wang X, Ge J, Li N (2019) Quercetin improves lipid metabolism via SCAP-SREBP2-LDLr signaling pathway in early stage diabetic nephropathy. Diabetes Metab Syndr Obes 12:827–839. https://doi.org/10.2147/DMSO.S195456
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no potential interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, L., Lan, S. Interference of S100A16 suppresses lipid accumulation and inflammation in high glucose-induced HK-2 cells. Int Urol Nephrol 53, 1255–1263 (2021). https://doi.org/10.1007/s11255-020-02731-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02731-4