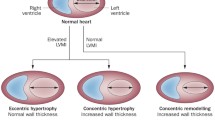
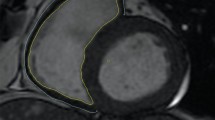
Abstract
Cardiovascular disease is the leading cause of death in patients with kidney failure or on chronic dialysis. Patients on chronic dialysis have a 10- to 50-fold increased risk of sudden cardiac death compared to patients with normal kidney function. Adverse changes in cardiac structure and function may not manifest with clinical symptoms in patients with kidney failure and, therefore, pose a challenge in identifying cardiac dysfunction early. Fortunately, there are multi-modality cardiac imaging techniques available, including echocardiography and cardiac magnetic resonance imaging, that can help our understanding of the pathophysiology of cardiac dysfunction in kidney failure. This review describes the benefits and limitations of these two commonly available cardiac imaging modalities to assess cardiac structure and function, thereby aiding nephrologists in choosing the most appropriate investigative tool based on individual clinical circumstances. For the purposes of this review, cardiac imaging for detection of coronary artery disease has been omitted.





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Johnson DW et al (2009) Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol 4(10):1620–1628
Herzog CA (2003) Cardiac arrest in dialysis patients: approaches to alter an abysmal outcome. Kidney Int 63:S197–S200
Green D et al (2011) Sudden cardiac death in hemodialysis patients: an in-depth review. Am J Kidney Dis 57(6):921–929
Marwick TH (2015) The role of echocardiography in heart failure. J Nucl Med 56(Suppl 4):31S–38S
Turakhia MP et al (2018) Chronic kidney disease and arrhythmias: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Eur Heart J 39(24):2314–2325
Foley RN et al (1995) Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47(1):186–192
Krishnasamy R et al (2015) Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS ONE 10(5):e0127044
Yamada S et al (2010) Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin J Am Soc Nephrol 5(10):1793–1798
Lang RM et al (2015) Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39.e14
Porter TR et al (2018) Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American society of echocardiography guidelines update. J Am Soc Echocardiogr 31(3):241–274
Mulvagh SL et al. (2008) American society of echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr 21(11): p. 1179–201; quiz 1281.
Chiu DYY et al (2015) Cardiac imaging in patients with chronic kidney disease. Nat Rev Nephrol 11(4):207–220
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100(21):1673–1680
Ie EHY, Zietse R (2006) Evaluation of cardiac function in the dialysis patient—a primer for the non-expert. Nephrol Dial Transpl 21(6):1474–1481
Bos WJ et al (2000) Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis 35(5):819–826
McIntyre CW (2009) Effects of hemodialysis on cardiac function. Kidney Int 76(4):371–375
Burton JO et al (2009) Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 4(5):914–920
McIntyre CW et al (2008) Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3(1):19–26
Sengelov M et al (2015) Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 8(12):1351–1359
Cikes M, Solomon SD (2015) Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 37(21):1642–1650
Lopez-Candales A, Hernandez-Suarez DF (2017) Strain imaging echocardiography: what imaging cardiologists should know. Curr Cardiol Rev 13(2):118–129
Green D, Kalra PR, Kalra PA (2012) Echocardiographic abnormalities in dialysis patients with normal ejection fraction. Nephrol Dial Transplant 27(12):4256–4259
Buckberg G et al (2008) Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation 118(24):2571–2587
Glassock RJ, Pecoits-Filho R, Barberato SH (2009) Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol 4(Supplement 1):S79–S91
Nagueh SF et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 29(4):277–314
Oh Jae K, Park S-J, Nagueh Sherif F (2011) Established and novel clinical applications of diastolic function assessment by echocardiography. Circulation 4(4):444–455
Badve SV et al (2016) The validity of left ventricular mass as a surrogate end point for all-cause and cardiovascular mortality outcomes in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis 68(4):554–563
de Roij van Zuijdewijn CLM et al (2015) Eccentric left ventricular hypertrophy and sudden death in patients with end-stage kidney disease. Am J Nephrol 42(2):126–133
Di Bella G et al (2014) The mosaic of the cardiac amyloidosis diagnosis: role of imaging in subtypes and stages of the disease. Euro Heart J Cardiovasc Imaging 15(12):1307–1315
Tsang W, Lang RM (2010) Echocardiographic evaluation of cardiac amyloid. Curr Cardiol Rep 12(3):272–276
Phelan D et al (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98(19):1442–1448
Urena P et al (1999) Evolutive aortic stenosis in hemodialysis patients: analysis of risk factors. Nephrologie 20(4):217–225
Zentner D et al (2011) Prospective evaluation of aortic stenosis in end-stage kidney disease: a more fulminant process? Nephrol Dial Transpl 26(5):1651–1655
London GM et al (2000) Calcification of the aortic valve in the dialyzed patient. J Am Soc Nephrol 11(4):778–783
Raggi P et al (2011) All-cause mortality in hemodialysis patients with heart valve calcification. Clin J Am Soc Nephrol 6(8):1990–1995
Ureña-Torres P et al (2019) Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dialysis Transpl. https://doi.org/10.1093/ndt/gfz133
Sise ME, Courtwright AM, Channick RN (2013) Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int 84(4):682–692
Hickson LJ et al (2016) Echocardiography criteria for structural heart disease in patients with end-stage renal disease initiating hemodialysis. J Am Coll Cardiol 67(10):1173–1182
Pabst S et al (2012) Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS ONE 7(4):e35310
Paneni F et al (2010) Right ventricular dysfunction in patients with end-stage renal disease. Am J Nephrol 32(5):432–438
Charytan DM et al (2014) Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol 176(1):99–109
Woolen SA et al (2020) Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Int Med 180(2):223–230
Perazella MA (2008) How should nephrologists approach gadolinium-based contrast imaging in patients with kidney disease?, Am Soc Nephrol.
Yee J (2017) Prophylactic hemodialysis for protection against gadolinium-induced nephrogenic systemic fibrosis: a Doll's House. Adv Chronic Kidney Di 24(3):133–135
Radenkovic D et al (2017) T(1) mapping in cardiac MRI. Heart Fail Rev 22(4):415–430
Edwards NC et al (2015) Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am J Cardiol 115(9):1311–1317
Sibley CT et al (2012) T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology 265(3):724–732
Kotecha T et al (2019) Acute changes in cardiac structural and tissue characterisation parameters following haemodialysis measured using cardiovascular magnetic resonance. Sci Rep 9(1):1388
Wali RK et al (2005) Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol 45(7):1051–1060
Baggiano A et al. (2020) noncontrast magnetic resonance for the diagnosis of cardiac amyloidosis. JACC. 13(1 Part 1): 69–80.
Acknowledgements
DP is a recipient of the Royal Australasian College of Physicians Jacquot Research Entry Scholarship 2019–2020.
Funding
No financial sponsor was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception, design, analysis, interpretation, drafting, critical revision and final approval of this version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The Better Evidence And Translation—Chronic Kidney Disease (BEAT-CKD) program is an independent collaborative research program funded by an Australian National Health and Medical Research Council (NHMRC) Program Grant (APP1092957) and supports research into kidney disease. DP is a recipient of the Royal Australasian College of Physicians Jacquot Research Entry Scholarship and has received speaking honoraria from the Australian Medical Forum. DJ is a current recipient of an Australian National Health and Medical Research Council Practitioner Fellowship. DJ has previously received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care. CH has received funding from Janssen and GlaxoSmithKline to her institution for trial steering committee roles and research grant support to her institution from Shire, Baxter, Fresenius, and Otsuka and travel sponsorship from Otsuka.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palamuthusingam, D., Reyaldeen, R., Johnson, D.W. et al. Assessment of cardiac structure and function in kidney failure: understanding echocardiography and magnetic resonance imaging for the nephrologist. Int Urol Nephrol 53, 699–712 (2021). https://doi.org/10.1007/s11255-020-02610-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02610-y