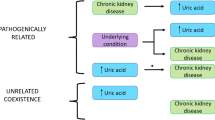
Abstract
Chronic kidney disease is prevalent, affecting more than one in ten adults. In this population, metabolic acidosis is considered a key underlying pathophysiological feature, tying together bone mineral disorders, sarcopenia, insulin resistance, vascular calcification, pro-inflammatory and pro-thrombotic states. This review aims to address the paucity of literature on alkalinizing agents, a promising treatment option that has known adverse effects.


Similar content being viewed by others
References
Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS et al (2016) Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE 11(7):e0158765
Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN et al (2015) A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88(5):950–957
Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW et al (2017) The systemic nature of CKD. Nat Rev Nephrol 13(6):344–358
Vanholder R, Fouque D, Glorieux G, Heine GH, Kanbay M, Mallamaci F et al (2016) Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diabetes Endocrinol 4(4):360–373
Kraut JA, Madias NE (2016) Metabolic acidosis of CKD: an update. Am J Kidney Dis 67(2):307–317
Abramowitz MK (2018) Metabolic acidosis and cardiovascular disease risk in CKD. Clin J Am Soc Nephrol 13(10):1451–1452
Raphael KL (2019) Metabolic acidosis in CKD: core curriculum 2019. Am J Kidney Dis 74(2):263–275
Navaneethan SD, Shao J, Buysse J, Bushinsky DA (2019) Effects of treatment of metabolic acidosis in CKD: a systematic review and meta-analysis. Clin J Am Soc Nephrol 14(7):1011–1020
Crimi E, Taccone FS, Infante T, Scolletta S, Crudele V, Napoli C (2012) Effects of intracellular acidosis on endothelial function: an overview. J Crit Care 27(2):108–118
Goligorsky MS (2015) Pathogenesis of endothelial cell dysfunction in chronic kidney disease: a retrospective and what the future may hold. Kidney Res Clin Pract 34(2):76–82
Nath KA, Hostetter MK, Hostetter TH (1985) Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 76(2):667–675
Wesson DE, Simoni J, Broglio K, Sheather S (2011) Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300(4):F830–F837
Kanbay M, Afsar B, Siriopol D, Unal HU, Karaman M, Saglam M et al (2016) Relevance of uric acid and asymmetric dimethylarginine for modeling cardiovascular risk prediction in chronic kidney disease patients. Int Urol Nephrol 48(7):1129–1136
Kanbay M, Yilmaz MI, Sonmez A, Turgut F, Saglam M, Cakir E et al (2011) Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol 33(4):298–304
Kanbay M, Afsar B, Gusbeth-Tatomir P, Covic A (2010) Arterial stiffness in dialysis patients: where are we now? Int Urol Nephrol 42(3):741–752
Rossi GP, Seccia TM, Barton M, Danser AHJ, de Leeuw PW, Dhaun N et al (2018) Endothelial factors in the pathogenesis and treatment of chronic kidney disease Part I: general mechanisms: a joint consensus statement from the European Society of Hypertension Working Group on Endothelin and Endothelial Factors and The Japanese Society of Hypertension. J Hypertens 36(3):451–461
Rossi GP, Seccia TM, Barton M, Danser AHJ, de Leeuw PW, Dhaun N et al (2018) Endothelial factors in the pathogenesis and treatment of chronic kidney disease Part II: role in disease conditions: a joint consensus statement from the European Society of Hypertension Working Group on Endothelin and Endothelial Factors and the Japanese Society of Hypertension. J Hypertens 36(3):462–471
Perticone F, Maio R, Tripepi G, Zoccali C (2004) Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation 110(7):821–825
Mervaala E, Finckenberg P, Lapatto R, Müller DN, Park JK, Dechend R et al (2003) Lipoic acid supplementation prevents angiotensin II-induced renal injury. Kidney Int 64(2):501–508
Himmelfarb J, Ikizler TA, Ellis C, Wu P, Shintani A, Dalal S et al (2014) Provision of antioxidant therapy in hemodialysis (PATH): a randomized clinical trial. J Am Soc Nephrol 25(3):623–633
Kendrick J, Shah P, Andrews E, You Z, Nowak K, Pasch A et al (2018) Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: a pilot randomized cross-over study. Clin J Am Soc Nephrol CJASN 13(10):1463–1470
Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS (2020) Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant 35(1):121–129
Sag AA, Covic A, London G, Vervloet M, Goldsmith D, Gorriz JL et al (2016) Clinical imaging of vascular disease in chronic kidney disease. Int Urol Nephrol 48(6):827–837
Kanbay M, Vervloet M, Cozzolino M, Siriopol D, Covic A, Goldsmith D et al (2017) Novel faces of fibroblast growth factor 23 (FGF23): iron deficiency, inflammation, insulin resistance, left ventricular hypertrophy, proteinuria and acute kidney injury. Calcif Tissue Int 100(3):217–228
Hénaut L, Chillon JM, Kamel S, Massy ZA (2018) Updates on the mechanisms and the care of cardiovascular calcification in chronic kidney disease. Semin Nephrol 38(3):233–250
Kanbay M, Goldsmith D, Akcay A, Covic A (2009) Phosphate - the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif 27(2):220–230
Lang F, Leibrock C, Pelzl L, Gawaz M, Pieske B, Alesutan I et al (2018) therapeutic interference with vascular calcification-lessons from klotho-hypomorphic mice and beyond. Front Endocrinol (Lausanne) 9:207
Zhang S, Xu J, Feng Y, Zhang J, Cui L, Zhang H et al (2018) Extracellular acidosis suppresses calcification of vascular smooth muscle cells by inhibiting calcium influx via L-type calcium channels. Clin Exp Hypertens 40(4):370–377
Mendoza F, Lopez I, De Oca AM, Perez J, Rodriguez M, Aguilera-Tejero E (2008) Metabolic acidosis inhibits soft tissue calcification in uremic rats. Kidney Int 73(4):407–414
Lomashvili K, Garg P, O’neill W (2006) Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int 69(8):1464–1470
Abramowitz MK (2017) Bicarbonate balance and prescription in ESRD. J Am Soc Nephrol 28(3):726–734
Căpuşă C, Ştefan G, Stancu S, Lipan M, Tsur LD, Mircescu G (2017) Metabolic acidosis of chronic kidney disease and subclinical cardiovascular disease markers: friend or foe? Medicine (Baltimore) 96(47):e8802
Aigner C, Cejka D, Sliber C, Fraunschiel M, Sunder-Plassmann G, Gaggl M (2019) Oral sodium bicarbonate supplementation does not affect serum calcification propensity in patients with chronic kidney disease and chronic metabolic acidosis. Kidney Blood Press Res 44(2):188–199
Mitchell JH, Wildenthal K, Johnson RL Jr (1972) The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int 1(5):375–389
Pedoto A, Caruso JE, Nandi J, Oler A, Hoffmann SP, Tassiopoulos AK et al (1999) Acidosis stimulates nitric oxide production and lung damage in rats. Am J Respir Crit Care Med 159(2):397–402
Fernandes D, Assreuy J (2008) Nitric oxide and vascular reactivity in sepsis. Shock 30(Suppl 1):10–13
Stancu S, Mircescu G, Mocanu A, Capusa C, Stefan G (2018) Metabolic acidosis of chronic kidney disease and cardiovascular disorders. Maedica 13(4):267–272
Taylor EN, Forman JP, Farwell WR (2007) Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension 50(2):320–324
Yilmaz MI, Solak Y, Covic A, Goldsmith D, Kanbay M (2011) Renal anemia of inflammation: the name is self-explanatory. Blood Purif 32(3):220–225
Tripepi G, Mallamaci F, Zoccali C (2005) Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 16(Suppl 1):S83–S88
Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R et al (2006) Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47(1):139–148
Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M et al (2012) Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7(12):1938–1946
Kanbay M, Onal EM, Afsar B, Dagel T, Yerlikaya A, Covic A et al (2018) The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol 50(8):1453–1466
Onal EM, Afsar B, Covic A, Vaziri ND, Kanbay M (2019) Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens Res 42(2):123–140
Ray SC, Baban B, Tucker MA, Seaton AJ, Chang KC, Mannon EC et al (2018) Oral NaHCO(3) activates a splenic anti-inflammatory pathway: evidence that cholinergic signals are transmitted via mesothelial cells. J Immunol 200(10):3568–3586
Ori Y, Zingerman B, Bergman M, Bessler H, Salman H (2015) The effect of sodium bicarbonate on cytokine secretion in CKD patients with metabolic acidosis. Biomed Pharmacother 71:98–101
Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H et al (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79(12):1370–1378
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T et al (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121(11):4393–4408
Melamed ML, Buttar RS, Coco M (2016) CKD-mineral bone disorder in stage 4 and 5 CKD: what we know today? Adv Chron Kidney Dis 23(4):262–269
Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P et al (2009) Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 4(1):186–194
KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009(113):S1–130
Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, Scolapio J et al (2009) Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case-control study. Pancreas 38(6):672–675
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15(8):2208–2218
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71(1):31–38
de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG et al (2011) Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol 6(9):2141–2149
Rebholz CM, Grams ME, Lutsey PL, Hoofnagle AN, Misialek JR, Inker LA et al (2016) Biomarkers of vitamin D status and risk of ESRD. Am J Kidney Dis 67(2):235–242
Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Navaneethan SD (2011) Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 6(1):50–62
Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E et al (2012) Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J 164(6):902–9.e2
Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH et al (2014) Effect of paricalcitol on left ventricular mass and function in CKD—the OPERA trial. J Am Soc Nephrol 25(1):175–186
Banerjee D, Chitalia N, Ster IC, Appelbaum E, Thadhani R, Kaski JC et al (2019) Impact of Vitamin D on Cardiac structure and function in CKD patients with hypovitaminosis D, a randomised controlled trial and meta-analysis. Eur Heart J Cardiovasc Pharmacother
Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H et al (2012) Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307(7):674–684
Charytan C, Coburn JW, Chonchol M, Herman J, Lien YH, Liu W et al (2005) Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis 46(1):58–67
Chonchol M, Locatelli F, Abboud HE, Charytan C, de Francisco AL, Jolly S et al (2009) A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis 53(2):197–207
Isakova T, Gutiérrez OM, Chang Y, Shah A, Tamez H, Smith K et al (2009) Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20(2):388–396
Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM et al (2012) Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23(8):1407–1415
Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M et al (2013) Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet 382(9900):1268–1277
Krieger NS, Sessler NE, Bushinsky DA (1992) Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol 262(3 Pt 2):F442–F448
Kraut JA, Mishler DR, Singer FR, Goodman WG (1986) The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int 30(5):694–700
Kraut JA (2000) Disturbances of acid-base balance and bone disease in end-stage renal disease. Semin Dial 13(4):261–266
Lin YF, Shieh SD, Diang LK, Lin SH, Chyr SH, Li BL et al (1994) Influence of rapid correction of metabolic acidosis on serum osteocalcin level in chronic renal failure. Asaio J 40(3):M440–M444
Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D (2006) Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail 28(1):1–5
McSherry E, Morris RC Jr (1978) Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest 61(2):509–527
Sebastian A, Morris RC Jr (1994) Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 331(4):279
Karet FE (2002) Inherited distal renal tubular acidosis. J Am Soc Nephrol 13(8):2178–2184
Karunarathne S, Udayakumara Y, Govindapala D, Fernando H (2012) Medullary nephrocalcinosis, distal renal tubular acidosis and polycythaemia in a patient with nephrotic syndrome. BMC Nephrol 13(1):66
Both T, Zietse R, Hoorn EJ, van Hagen PM, Dalm VA, van Laar JA et al (2014) Everything you need to know about distal renal tubular acidosis in autoimmune disease. Rheumatol Int 34(8):1037–1045
Peter WLS, Wazny LD, Weinhandl E, Cardone KE, Hudson JQ (2017) A review of phosphate binders in chronic kidney disease: incremental progress or just higher costs? Drugs 77(11):1155–1186
Suki WN, Zabaneh R, Cangiano J, Reed J, Fischer D, Garrett L et al (2007) Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72(9):1130–1137
Foley RN, Wang C, Ishani A, Collins AJ, Murray AM (2007) Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 27(3):279–286
Souza VA, Oliveira D, Mansur HN, Fernandes NM, Bastos MG (2015) Sarcopenia in chronic kidney disease. J Bras Nefrol 37(1):98–105
Workeneh BT, Mitch WE (1128S) Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 91(4):1128S–S1132
Lai S, Muscaritoli M, Andreozzi P, Sgreccia A, De Leo S, Mazzaferro S et al (2019) Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition 62:108–114
Abramowitz MK, Hostetter TH, Melamed ML (2011) Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am J Kidney Dis 58(1):29–38
Abramowitz MK, Hostetter TH, Melamed ML (2012) Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int 81(10):1033–1042
Wang XH, Mitch WE (2014) Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10(9):504–516
Hamm LL (1999) Role of glucocorticoids in acidosis. Am J Kidney Dis 34(5):960–965
Esche J, Shi L, Sánchez-Guijo A, Hartmann MF, Wudy SA, Remer T (2016) Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int 90(2):325–333
Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J (2002) Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int 61(4):1286–1292
Stein A, Moorhouse J, Iles-Smith H, Baker F, Johnstone J, James G et al (1997) Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney Int 52(4):1089–1095
Verove C, Maisonneuve N, El Azouzi A, Boldron A, Azar R (2002) Effect of the correction of metabolic acidosis on nutritional status in elderly patients with chronic renal failure. J Renal Nutr 12(4):224–228
de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM (2009) Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20(9):2075–2084
Abramowitz MK (2014) Acid-base balance and physical function. Clin J Am Soc Nephrol 9(12):2030–2032
Watson EL, Kosmadakis GC, Smith AC, Viana JL, Brown JR, Molyneux K et al (2013) Combined walking exercise and alkali therapy in patients with CKD4-5 regulates intramuscular free amino acid pools and ubiquitin E3 ligase expression. Eur J Appl Physiol 113(8):2111–2124
Makanae Y, Fujita S (2015) Role of exercise and nutrition in the prevention of sarcopenia. J Nutr Sci Vitaminol 61(Supplement):S125–S127
Maykish A, Sikalidis AK (2020) Utilization of hydroxyl-methyl butyrate, leucine, glutamine and arginine supplementation in nutritional management of sarcopenia—implications and clinical considerations for Type 2 diabetes mellitus risk modulation. J Person Med 10(1):19
Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E et al (1998) Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int 53(5):1343–1347
Spoto B, Pisano A, Zoccali C (2016) Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 311(6):F1087–F1108
Liao MT, Sung CC, Hung KC, Wu CC, Lo L, Lu KC (2012) Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol 2012:691369
Landau M, Kurella-Tamura M, Shlipak MG, Kanaya A, Strotmeyer E, Koster A et al (2011) Correlates of insulin resistance in older individuals with and without kidney disease. Nephrol Dial Transplant 26(9):2814–2819
Walker BG, Phear DN, Martin FI, Baird CW (1963) inhibition of insulin by acidosis. Lancet 2(7315):964–965
Shechter Y, Ron A (1986) Effect of depletion of bicarbonate or phosphate ions on insulin action in rat adipocytes. Further characterization of the receptor-effector system. J Biol Chem. 261(32):14951–4
Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T (2005) Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis 45(2):275–280
Kobayashi S, Maejima S, Ikeda T, Nagase M (2000) Impact of dialysis therapy on insulin resistance in end-stage renal disease: comparison of haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 15(1):65–70
Bellasi A, Di Micco L, Santoro D, Marzocco S, De Simone E, Cozzolino M et al (2016) Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol 17(1):158
Chen W, Abramowitz MK (2014) Treatment of metabolic acidosis in patients with CKD. Am J Kidney Dis 63(2):311–317
Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE (2010) Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78(3):303–309
Chen W, Abramowitz MK (2017) Epidemiology of acid-base derangements in CKD. Adv Chron Kidney Dis 24(5):280–288
Kirschbaum B (2000) Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis 35(6):1068–1071
Goraya N, Simoni J, Jo CH, Wesson DE (2014) Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86(5):1031–1038
Goraya N, Simoni J, Jo C, Wesson DE (2012) Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int 81(1):86–93
Goraya N, Simoni J, Jo CH, Wesson DE (2013) A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8(3):371–381
Acknowledgements
MK gratefully acknowledge use of the services and facilities of the Koç University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.”
Funding
This study was not funded by any grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Copur, S., Sag, A.A., Afsar, B. et al. Complications of metabolic acidosis and alkalinizing therapy in chronic kidney disease patients: a clinician-directed organ-specific primer. Int Urol Nephrol 52, 2311–2320 (2020). https://doi.org/10.1007/s11255-020-02563-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02563-2