Abstract
Purpose
MRI-targeted biopsy has improved prostate biopsy yield. However, cost constraints have made it difficult for many institutions to implement the newer methods. We evaluated the performance of a low-cost cognitive-targeting biopsy protocol based on 1.5 T multiparametric MRI graded with Prostate Imaging Reporting and Data System (PI-RADS) version 2 to examine the role for these institutions moving forward.
Methods
Retrospective analysis of 251 consecutive patients with prostate-specific antigen (PSA) under 50 who underwent MRI and subsequent prostate biopsy at a single facility. In addition to systematic biopsy, targeted cores were obtained with cognitive recognition under ultrasound. A control group of 267 consecutive patients with PSA under 50 biopsied without prior MRI was analyzed.
Results
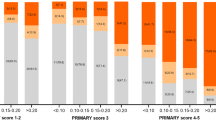
Prostate biopsy preceded by MRI had a significantly higher probability of detecting both prostate cancer (68.1% vs. 51.3%) and clinically significant prostate cancer (57.4% vs. 39.7%) (p values < 0.01). Combination of systematic and targeted biopsy outperformed either regimen alone. PSA density and PI-RADS score were identified as independent risk factors, and a proposed diagnostic model (PSA density ≥ 0.25 or PI-RADS score ≥ 4) showed sensitivity of 88.6%, specificity of 55%, PPV of 81.2%, NPV of 68.8%, and accuracy of 78.0%.
Conclusions
Both pre-biopsy MRI and cognitive-targeted biopsy contributed to improvement of cancer yield. Future alterations of possible benefit included increasing target cores per lesion, and combining PI-RADS score and PSA density as indicators for biopsy. Similar protocols may represent an on-going role for lower volume centers in the diagnosis of prostate cancer.

Similar content being viewed by others
References
Singh H, Canto L, Shariat SF et al (2004) Predictors of prostate cancer after initial negative systematic 12 core biopsy. J Urol 171:1850–1854
Keetch DW, Catalona WJ, Smith DS (1994) Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol 151:1571–1574
Draisma G, Etxioni R, Tsodikov A et al (2009) Lead time and overdiagnosis in protate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 101:374–383
Loeb S, Vellekoop A, Ahmed HU et al (2013) Systematic review of complications of prostate biopsy. Eur Urol 64:876–892
Hoeks CM, Barentsz JO, Hambrock T et al (2011) Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 261:46–66
An JY, Sidana A, Holzman SA et al (2018) Ruling out clinically significant prostate cancer with negative multi-parametric MRI. Int Urol Neph 50:7–12
Barentsz JO, Richenburg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757
Barentsz JO, Weinreb JC, Verma S et al (2016) Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol 69:41–49
Peuch P, Ouzzane A, Gailard V et al (2014) Multiparametric MRI-Targeted TRUS prostate biopsies using visual registration. Res Int, Biomed. https://doi.org/10.1155/2014/819360
Schiavina R, Vagnoni V, D’Agostino D et al (2017) “In-bore” MRI-guided prostate biopsy using an endorectal nonmagnetic device: a prospective study of 70 consecutive patients. Clin Genitourin Cancer 15:417–427
Hakozaki Y, Matsushima H, Kumagai J et al (2017) A prospective study of magnetic resonance imaging and ultrasonography (MRI/US)-fusion targeted biopsy and concurrent systematic transperineal biopsy with the average of 18-cores to detect clinically significant prostate cancer. BMC Urol 17:117
Venderlink W, van Luijtelaar A, Bomers JG et al (2017) Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol 73:353–360
Borkowetz A, Platzek I, Toma M et al (2017) Evaluation of prostate imaging reporting and data classification in the prediction of tumor aggressiveness in targeted magnetic resonance/ultrasound-fusion biopsy. Urol Int 99:177–185
Maxeiner A, Kittner B, Blobel C et al (2018) Primary magnetic resonance imaging/ultrasonography fusion-guided biopsy of the prostate. BJU Int 122:211–218
Mannaerts CK, Kajtazovic A, Lodeizen OAP et al (2019) The added value of systematic biopsy in men with suspicion of prostate cancer undergoing multiparametric MRI-targeted biopsy. Urol Oncol 37(298):e1–e9
Hansen NL, Kesch C, Barrett T et al (2017) Multicentre evauation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int 120:631–638
Peuch P, Rouviere O, Renard-Penna R et al (2013) Multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy—prospective multicenter study. Radiology 268:461–469
Wysock JS, Rosenkratz AB, Huang WC et al (2014) A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS Trial. Eur Urol 66:343–351
Schimmoller L, Quentin M, Blondin D et al (2016) Targeted MRI-guided prostate biopsy: are two biopsy cores per MRI-lesion required? Eur Radiol 26:3858–3864
Rosenkratz AB, Verma S, Choyke P et al (2016) Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol 196:1613–1618
Bhat NR, Vetter JM, Andriole GL, Shetty AS, Ippolito JE, Kim EH (2018) Magnetic resonance imaging-defined prostate-specific antigen density significantly improves the risk prediction for clinically significant prostate cancer on biopsy. J Urol 126:152–157
Distler FA, Radtke JP, Bonekamp D et al (2017) The value of PSA density in combination with PI-RADS™ for the accuracy of prostate cancer prediction. J Urol 198:575–582
Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM (2015) Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol 67:1112–1121
Zhang L, Tang M, Chen S, Lei X, Zhang X, Huan Y (2017) A meta-analysis of use of prostate imaging reporting and data system version 2 (PI-RADS V2) with multiparametric MR imaging for the detection of prostate cancer. Eur Radiol 27:5204–5214
Cuocolo R, Stanzione A, Rusconi G et al (2018) PSA-density does not improve bi-parametric prostate MR detection of prostate cancer in a biopsy naïve population. Eur J Radiol 104:64–70
Ahmed HU, Bosaily AE, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822
Kryvenko ON, Carter HB, Trock BJ, Epstein JI (2014) Biopsy criteria for determining appropriateness for active surveillance in the modern era. Urology 83:869–874
Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V (2017) Cost-effectiveness of MR imaging-guided strategies for detection of prostate cancer in biopsy-naïve men. Radiology 285:157–166
Altok M, Kim B, Patel BB et al (2018) Cost and efficacy comparison of five prostate biopsy modalities: a platform for integrating cost into novel-platform comparative research. Prostate Cancer Prostatic Dis 21:524–532
Hutchinson RC, Costa DN, Lotan Y (2016) The economic effect of using magnetic resonance imaging and magnetic resonance ultrasound fusion biopsy for prostate cancer diagnosis. Urol Oncol 34:296–302
Kaufmann S, Russo GI, Bamberg F et al (2018) Prostate cancer detection in patients with prior negative biopsy undergoing cognitive-, robotic- or in-bore MRI target biopsy. World J Urol 36:761–768
Osses DF, van Asten JJ, Tijsterman JD (2017) Cognitive-targeted versus magnetic resonance Imaging Guided prostate biopsy in prostate cancer detection. Curr Urol 11:182–188
Yaxley AJ, Yaxley JW, Thangasamy IA, Ballard E, Pokorny MR (2017) Comparison between target magnetic resonance imaging (MRI) in-gantry and cognitively directed transparietal or transrectal-guided prostate biopsies for Prostate Imaging-Reporting and Data System (PI-RADS) 3–5 MRI lesions. BJU Int 120(Suppl 3):43–50
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YuT was responsible for conception and design of the study, as well as acquisition and analysis of data, and drafted the manuscript. YoT oversaw the conception and design of the study, and supervised data acquisition and drafting of the manuscript. KT and SN contributed equally to YuT in the acquisition of data. EY served a primary role in interpretation of the data. HK provided supervision for the drafting of the manuscript, and a chief role in critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethics approval
Approval of the institutional review board of the Institute of Medical Science, University of Tokyo (Approval no. 2019-2-0607), and Musashino Red Cross Hospital (Approval no. 1025).
Consent to participate/consent for publication
All patients whose data were retrospectively analyzed were informed of the use of their clinical information under anonymization, and received an opportunity to object to use or publication.
Availability of data and material
Available from corresponding author upon reasonable request.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeshima, Y., Tanaka, Y., Takemura, K. et al. Evaluating the efficacy of a low-cost cognitive MRI-targeted prostate biopsy protocol: is there still a role for lower volume centers in the Prostate Imaging Reporting and Data System (PI-RADS) version 2 era?. Int Urol Nephrol 52, 2043–2050 (2020). https://doi.org/10.1007/s11255-020-02533-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02533-8