Abstract
Purpose
Uremic toxins produced by gut microbiota (indoxyl sulfate—IS, p-cresyl sulfate—p-CS, and indole-3-acetic acid—IAA) accumulate in hemodialysis (HD) patients and exhibit potent inflammatory effects. However, the impact of these toxins on nuclear E2-related factor 2 (Nrf2) and nuclear factor-kappa B (NF-κB) expression in HD patients remains poorly defined. The aim of this study was to evaluate the association between uremic toxins and Nrf2/NF-κB expression in vitro (RAW 264.7 macrophage-like cells) and in peripheral blood mononuclear cells from HD patients.
Methods
Uremic toxins, C-reactive protein (CRP), interleukin-6 (IL-6) and malondialdehyde (MDA) levels were measured in fifteen HD patients and nine healthy individuals. RAW 264.7 macrophage-like cells were incubated with IS, as a prototype of protein-bound uremic toxin. Nrf2 and NF-κB expressions were analyzed by RT-qPCR.
Results
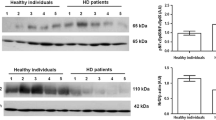
HD patients presented high levels of inflammatory markers, MDA and uremic toxins. In addition, they presented high NF-κB and low Nrf2 expression. Uremic toxins were positively correlated with NF-κB expression (IS, ρ = 0.58, p < 0.003; p-CS, ρ = 0.71, p < 0.001; IAA, ρ = 0.62, p < 0.001) and negatively with Nrf2 (IS, ρ = − 0.48, p = 0.01; p-CS, ρ = − 0.46, p < 0.02). Uremic toxins also exhibited positive correlations with CRP and MDA levels. Multivariate analysis revealed that p-CS is a determinant factor of NF-κB expression. In RAW 264.7 culture, NF-κB mRNA expression was stimulated by IS, while Nrf2 was downregulated.
Conclusions
Thus, uremic toxins may stimulate NF-κB mRNA and decrease Nrf2 expression in HD patients and, consequently, trigger inflammation and oxidative stress.




Similar content being viewed by others
References
Meijers BK, Evenepoel P (2011) The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant 26:759–761
Mafra D, Lobo JC, Barros AF, Koppe L, Vaziri ND, Fouque D (2014) Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol 9(3):399–410
Deltombe O, Biesen WV, Glorieux G, Massy Z, Dhondt A, Eloot S (2015) Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins 7:3933–3946
Stockler-Pinto MB, Saldanha JF, Yi D, Mafra D, Fouque D, Soulage CO (2016) The uremic toxin indoxyl sulfate exacerbates reactive oxygen species production and inflammation in 3T3-L1 adipose cells. Free Radic Res 50(3):337–344
Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S (2014) The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins 6:934–949
Lee WC, Li LC, Chen JB, Chang HW (2015) Indoxyl sulfate-induced oxidative stress, mitochondrial dysfunction, and impaired biogenesis are partly protected by vitamin C and N-acetylcysteine. Sci World J 2015:1–6
Tang WH, Wang CP, Chung FM, Huang LL, Yu TH, Hung WC et al (2015) Uremic retention solute indoxyl sulfate level is associated with prolonged QTc interval in early CKD patients. PLoS ONE 10(3):e0119545
Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P et al (2015) The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol 27(5):1389–1399
Gao C, Ji S, Dong W, Qi Y, Song W, Cui D, Shi J (2015) Indolic uremic solutes enhance procoagulant activity of red blood cells through phosphatidylserine exposure and microparticle release. Toxins 7:4390–4403
Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Vanholder R, Brunet P (2009) Protein-bound toxin—update 2009. Semin Dial 22(4):334–339
Rossi M, Campbell KL, Johnson DW, Staton T, Vesey DA, Coombes JS et al (2014) Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3–4 chronic kidney disease. Arch Med Res 45(4):309–317
Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M et al (2013) p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int 83:582–592
Dou L, Sallée M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N (2014) The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 26(4):876–887
Bolati D, Shimizu H, Yisireyili M, Nishijima F, Niwa T (2013) Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-kappaB. BMC Nephrol 4(14):56
Pedruzzi LM, Stockler-Pinto MB Jr, Leite M, Mafra D (2012) Nrf2-keap1 system versus NF-kappaB: the good and the evil in chronic kidney disease? Biochimie 94:2461–2466
Leal VO, Saldanha JF, Stockler-Pinto MB, Cardozo LF, Santos FR, Albuquerque AS et al (2015) NRF2 and NF-κB mRNA expression in chronic kidney disease: a focus on nondialysis patients. Int Urol Nephrol 47(12):1985–1989
Daugirdas JT (1993) Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol 4:1205–1213
de Loor H, Meijers BK, Meyer TW, Bammens B, Verbeke K, Dehaen W, Evenepoiel P (2009) Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J Chromatogr A 1216(22):4684–4688
Meert N, Schepers E, Glorieux G, Landschoot MV, Goeman JL, Waterloos MA et al (2012) Novel method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: clinical data and pathophysiological implications. Nephrol Dial Transplant 27(6):2388–2396
Boelaert J, Lynen F, Glorieux G, Eloot S, Van Landschoot M, Waterloos MA et al (2013) A novel UPLC-MS-MS method for simultaneous determination of seven uremic retention toxins with cardiovascular relevance in chronic kidney disease patients. Anal Bioanal Chem 405(6):1937–1947
Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM et al (2007) Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal 43(2):619–624
Neirynck N, Vanholder R, Schepers E, Eloot S, Pletinck A, Glorieux G (2013) An update on uremic toxins. Int Urol Nephrol 45:139–150
Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E (2010) Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25(4):1183–1191
Lin CJ, Wu V, Wu PC (2015) Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 10(7):e0132589
Ito S, Osaka M, Higuchi Y, Nishijima F, Ishii H, Yoshida M (2010) Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J Biol Chem 285(50):38869–38875
Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T (2012) Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Ren Nutr 22(1):86–89
Adesso S, Popolo A, Bianco G, Sorrentino R, Pinto A, Autore G et al (2013) The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS One 8(9):e76778.30
Shimizu H, Yisireyli M, Higashiyama Y, Nishijima F, Niwa T (2013) Indoxyl sulfate upregulates renal expression of ICAM-1 via production of ROS and activation of NF-kappa b and p53 in proximal tubular cell. Life Sci 92(2):1143–1148
Masai N, Tatebe J, Yoshino G, Morita T (2010) Indoxyl sulfate stimulates monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells by inducing oxidative stress through activation of the NADPH oxidase-nuclear factor-kB pathway. Circ J 74:2216–2224
Gelasco AK, Raymond JR (2006) Indoxyl sulfate induces complex redox alterations in mesangial cells. Am J Physiol Ren Physiol 290:1551–1558
Goundouin B, Cerini C, Dou L, Salée M, Durvai-Sabatier A, Pletinck A et al (2013) Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 84:733–744
Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H (2010) Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 31:1771–1779
Jung KA, Kwak MK (2010) The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 15(10):7266–7291
Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P (2010) Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res 44(11):1267–1288
Baird L, Dinkova-Kostov AT (2011) The cytoprotective role of the Keap1-Nrf2 pathway. Arch of Toxicol 85(4):241–272
Kim HJ, Vaziri ND (2010) Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Ren Physiol 98(3):662–671
Lau WL, Liu SM, Pahlevan S, Yuan J, Khazaeli M, Ni Z et al (2014) Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption. Dig Dis Sci 60(5):1215–1222
Koppe L, Mafra D, Fouque D (2015) Probiotics and chronic kidney disease. Kidney Int 88(5):958–966
Saito H, Yoshimura M, Saigo C, Komori M, Nomura Y, Yamamoto Y et al (2014) Hepatic sulfotransferase as a nephropreventing target by suppression of the uremic toxin indoxyl sulfate accumulation in ischemic acute kidney injury. Toxicol Sci 141(1):206–217
Gao H, Liu S (2017) Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci 185:23–29
Acknowledgements
This study was supported by Conselho Nacional de Pesquisa (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Clinical Research Unit (UPC) at Fluminense Federal University (UFF), Comité Français d´Evaluation de la Coopération Universitaire avec le Brésil (COFECUB), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Institut National de la Santé et de la Recherche Medicale (INSERM) and Institut National des Sciences Appliquées de Lyon (INSA-Lyon).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Medicine Faculty of Federal University Fluminense and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Statement of animal studies
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stockler-Pinto, M.B., Soulage, C.O., Borges, N.A. et al. From bench to the hemodialysis clinic: protein-bound uremic toxins modulate NF-κB/Nrf2 expression. Int Urol Nephrol 50, 347–354 (2018). https://doi.org/10.1007/s11255-017-1748-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1748-y