Abstract
Purpose
Hypoxia plays a significant role in the pathogenesis of acute kidney injury (AKI). Autophagy protects from AKI. Amino acid deprivation induces autophagy. The effect of l-tryptophan depletion on survival and autophagy in cultures of renal proximal tubular epithelial cells (RPTECs) under hypoxia was evaluated.
Methods
RPTECs were preconditioned in a medium containing or not tryptophan, following culture under hypoxia and treatment with or without the autophagy inhibitor chloroquine. Cell survival was assessed by cell imaging, the level of certain proteins by western blotting and cellular ATP fluorometrically.
Results
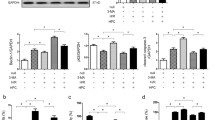
Preconditioning of RPTECs in a medium without tryptophan activated general control nonderepressible 2 kinase and induced changes that favored autophagy and cell survival under hypoxic conditions. Additionally, it increased cellular ATP, while it inhibited apoptosis. Inhibition of autophagy nullified the induced increase in cellular ATP and cell survival by the absence of tryptophan. The absence of tryptophan increased p53, although its effect on p53’s transcriptional targets was heterogeneous. In accordance with the decreased apoptosis, expression of p21 increased, while expression of Bax decreased. The expression of BNIP3L, which may be pro-apoptotic or pro-autophagic, increased. Considering the decreased apoptosis, it is likely that tryptophan depletion enhances autophagy through a p53-mediated increase of BNIP3L.
Conclusion
Preconditioning of primary human RPTECs in a medium without tryptophan increases their survival under hypoxia by inducing autophagy. Identifying new molecular mechanisms that protect renal tissue from hypoxia could be proved clinically important in the prevention of AKI.





Similar content being viewed by others
References
Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS (2007) Community-based incidence of acute renal failure. Kidney Int 72(2):208–212. doi:10.1038/sj.ki.5002297
Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR (2009) Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 53(6):961–973. doi:10.1053/j.ajkd.2008.11.034
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334(22):1448–1460. doi:10.1056/NEJM199605303342207
Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341(23):1725–1730. doi:10.1056/NEJM199912023412303
Shoskes DA, Halloran PF (1996) Delayed graft function in renal transplantation: etiology, management and long-term significance. J Urol 155(6):1831–1840
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121(11):4210–4221. doi:10.1172/JCI45161
Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM (2009) Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29(10):2570–2581. doi:10.1128/MCB.00166-09
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293. doi:10.1016/j.molcel.2010.09.023
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15(2):81–94. doi:10.1038/nrm3735
Kaushal GP, Shah SV (2016) Autophagy in acute kidney injury. Kidney Int 89(4):779–791. doi:10.1016/j.kint.2015.11.021
Jiang M, Liu K, Luo J, Dong Z (2010) Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 176(3):1181–1192. doi:10.2353/ajpath.2010.090594
Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y (2011) Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22(5):902–913. doi:10.1681/ASN.2010070705
Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z (2012) Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82(12):1271–1283. doi:10.1038/ki.2012.261
Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB (2012) Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8(5):826–837. doi:10.4161/auto.19419
Guan X, Qian Y, Shen Y, Zhang L, Du Y, Dai H, Qian J, Yan Y (2015) Autophagy protects renal tubular cells against ischemia/reperfusion injury in a time-dependent manner. Cell Physiol Biochem 36(1):285–298. doi:10.1159/000374071
Mei S, Livingston M, Hao J, Li L, Mei C, Dong Z (2016) Autophagy is activated to protect against endotoxic acute kidney injury. Sci Rep 6:22171. doi:10.1038/srep22171
Chaudhary K, Shinde R, Liu H, Gnana-Prakasam JP, Veeranan-Karmegam R, Huang L, Ravishankar B, Bradley J, Kvirkvelia N, McMenamin M, Xiao W, Kleven D, Mellor AL, Madaio MP, McGaha TL (2015) Amino acid metabolism inhibits antibody-driven kidney injury by inducing autophagy. J Immunol 194(12):5713–5724. doi:10.4049/jimmunol.1500277
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140(3):313–326. doi:10.1016/j.cell.2010.01.028
Russell RC, Yuan HX, Guan KL (2014) Autophagy regulation by nutrient signaling. Cell Res 24(1):42–57. doi:10.1038/cr.2013.166
Gallinetti J, Harputlugil E, Mitchell JR (2013) Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J 449(1):1–10. doi:10.1042/BJ20121098
B’Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A (2013) The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 41(16):7683–7699. doi:10.1093/nar/gkt563
Fougeray S, Mami I, Bertho G, Beaune P, Thervet E, Pallet N (2012) Tryptophan depletion and the kinase GCN2 mediate IFN-gamma-induced autophagy. J Immunol 189(6):2954–2964. doi:10.4049/jimmunol.1201214
Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD (2014) FGF21 is an endocrine signal of protein restriction. J Clin Invest 124(9):3913–3922. doi:10.1172/JCI74915
Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR (2012) Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med 4(118):118ra111. doi:10.1126/scitranslmed.3002629
Lieberthal W, Nigam SK (1998) Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol 275(5):F623–F631
Eleftheriadis T, Pissas G, Sounidaki M, Tsogka K, Antoniadis N, Antoniadi G, Liakopoulos V, Stefanidis I (2016) Indoleamine 2,3-dioxygenase, by degrading l-tryptophan, enhances carnitine palmitoyltransferase I activity and fatty acid oxidation, and exerts fatty acid-dependent effects in human alloreactive CD4+ T-cells. Int J Mol Med. doi:10.3892/ijmm.2016.2750
Staiger K, Staiger H, Weigert C, Haas C, Haring HU, Kellerer M (2006) Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes 55(11):3121–3126. doi:10.2337/db06-0188
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 258(6):479–517. doi:10.1111/j.1365-2796.2005.01570.x
Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F (2010) Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol 185(12):7349–7357. doi:10.4049/jimmunol.1000576
Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13(5):589–598. doi:10.1038/ncb2220
Hollinshead KE, Tennant DA (2016) Mitochondrial metabolic remodeling in response to genetic and environmental perturbations. Wiley Interdiscip Rev Syst Biol Med 8(4):272–285. doi:10.1002/wsbm.1334
Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515(7527):431–435. doi:10.1038/nature13909
Eleftheriadis T, Pissas G, Antoniadi G, Spanoulis A, Liakopoulos V, Stefanidis I (2014) Indoleamine 2,3-dioxygenase increases p53 levels in alloreactive human T cells, and both indoleamine 2,3-dioxygenase and p53 suppress glucose uptake, glycolysis and proliferation. Int Immunol 26(12):673–684. doi:10.1093/intimm/dxu077
Kwon NH, Kang T, Lee JY, Kim HH, Kim HR, Hong J, Oh YS, Han JM, Ku MJ, Lee SY, Kim S (2011) Dual role of methionyl-tRNA synthetase in the regulation of translation and tumor suppressor activity of aminoacyl-tRNA synthetase-interacting multifunctional protein-3. Proc Natl Acad Sci USA 108(49):19635–19640. doi:10.1073/pnas.1103922108
Brady CA, Attardi LD (2010) p53 at a glance. J Cell Sci 123(Pt 15):2527–2532. doi:10.1242/jcs.064501
Liu Y, Laszlo C, Liu Y, Liu W, Chen X, Evans SC, Wu S (2010) Regulation of G(1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation. Neoplasia 12(1):61–68
Xenaki G, Ontikatze T, Rajendran R, Stratford IJ, Dive C, Krstic-Demonacos M, Demonacos C (2008) PCAF is an HIF-1alpha cofactor that regulates p53 transcriptional activity in hypoxia. Oncogene 27(44):5785–5796. doi:10.1038/onc.2008.192
Fei P, Wang W, Kim SH, Wang S, Burns TF, Sax JK, Buzzai M, Dicker DT, McKenna WG, Bernhard EJ, El-Deiry WS (2004) Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell 6(6):597–609. doi:10.1016/j.ccr.2004.10.012
Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z (2008) Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74(5):631–640. doi:10.1038/ki.2008.214
Ellenbogen MA, Young SN, Dean P, Palmour RM, Benkelfat C (1996) Mood response to acute tryptophan depletion in healthy volunteers: sex differences and temporal stability. Neuropsychopharmacology 15(5):465–474. doi:10.1016/S0893-133X(96)00056-5
Funding
This study was funded only by the resources of our department
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable, since this article does not contain any studies with human participants.
Rights and permissions
About this article
Cite this article
Eleftheriadis, T., Pissas, G., Sounidaki, M. et al. Preconditioning of primary human renal proximal tubular epithelial cells without tryptophan increases survival under hypoxia by inducing autophagy. Int Urol Nephrol 49, 1297–1307 (2017). https://doi.org/10.1007/s11255-017-1596-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1596-9