Abstract
Introduction
The purpose of this study was to provide a comprehensive dataset of porcine urinary bladder smooth muscle properties. Particularly, the history dependence of force production, namely force depression (FD) following shortening and force enhancement (FE) following stretch, was analysed. During active micturition, the circumference of the urinary bladder changes enormously. Thus, FD might be an important phenomenon during smooth muscle contraction.
Materials and methods
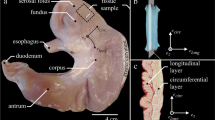
Electrically stimulated, intact urinary bladder strips from pigs (n = 10) were suspended in an aerated-filled organ bath, and different isometric, isotonic, and isokinetic contraction protocols were performed to determine the force–length and the force–velocity relation. FD and FE were assessed in concentric and eccentric contractions with different ramp lengths and ramp velocities.
Results
Bladder smooth muscles exhibit considerable amounts of FD and FE. The amount of FD increased significantly with ramp length, while FE did not change. However, FE and FD were independent of ramp velocity.
Conclusion
The results imply that smooth muscle bladder strips exhibit similar muscle properties and history-dependent behaviour compared to striated muscles. The provided dataset of muscle properties is important for bladder modelling as well as for the analyses and interpretation of dynamic bladder filling and voiding.






Similar content being viewed by others
Abbreviations
- Curv:
-
Curvature factor
- F im :
-
Maximum isometric muscle force
- FD:
-
Force depression
- FE:
-
Force enhancement
- L opt :
-
Optimum muscle length associated with F im
- L ramp :
-
Ramp length
- L s :
-
Slack length
- L start :
-
Start length
- V max :
-
Maximal shortening velocity
References
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc B Biol Sci 126:136–195
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Chem 7:255–318
Rode C, Siebert T, Tomalka A, Blickhan R (2016) Myosin filament sliding through the Z-disc relates striated muscle fibre structure to function. Proc Biol Sci 283:10–13
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117:77–86
Edman KA, Elzinga G, Noble MI (1982) Residual force enhancement after stretch of contracting frog single muscle fibers. J Gen Physiol 80:769–784
Lee HD, Herzog W (2002) Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J Physiol 545:321–330
Linari M, Lucii L, Reconditi M, Casoni MEV, Amenitsch H, Bernstorff S, Piazzesi G, Lombardi V (2000) A combined mechanical and X-ray diffraction study of stretch potentiation in single frog muscle fibres. J Physiol 526(3):589–596
De Ruiter CJ, De Haan A, Jones DA, Sargeant AJ (1998) Shortening-induced force depression in human adductor pollicis muscle. J Physiol 507:583–591
Herzog W, Leonard TR, Wu JZ (2000) The relationship between force depression following shortening and mechanical work in skeletal muscle. J Biomech 33:659–668
Rassier DE, Herzog W (2002) Force enhancement following an active stretch in skeletal muscle. J Electromyogr Kinesiol 12:471–477
Campbell SG, Campbell KS (2011) Mechanism of residual force enhancement in skeletal muscle: insights from experiments and mathematical models. Biophys Rev 3:199–207
Siebert T, Rode C (2014) Computational modeling of muscle biomechanics. In: Jin Z (ed) Computational modelling of biomechanics and biotribology in the musculoskeletal system, 1st edn. Woodhead Publishing/Elsevier, Amsterdam, pp 173–243
Rassier DE, Herzog W (2004) Effects of shortening on stretch-induced force enhancement in single skeletal muscle fibers. J Biomech 37:1305–1312
Rode C, Siebert T, Blickhan R (2009) Titin-induced force enhancement and force depression: A “sticky-spring” mechanism in muscle contractions? J Theor Biol 259:350–360
Edman K (1993) Depression of tetanic force induced by loaded shortening of frog muscle fibres. J Physiol 466:535–552
Van Asselt E, Pel JJM, van Mastrigt R (2007) Shortening induced effects on force (re)development in pig urinary smooth muscle. J Biomech 40:1534–1540
Griffiths DJ, van Mastrigt R, van Duyl WA, Coolseat BLRA (1979) Active mechanical properties of the smooth muscle of the urinary bladder. Med Biol Eng Comput 17:281–290
Uvelius B (2001) Length–tension relations of in vitro urinary bladder smooth muscle strips. J Pharmacol Toxicol Methods 45:87–90
Van Mastrigt R (2002) Mechanical properties of (urinary bladder) smooth muscle. J Muscle Res Cell Motil 23:53–57
Van Mastrigt R, Glerum JJ (1985) In vitro comparison of isometric and stop-test contractility parameters for the urinary bladder. Urol Res 13:11–17
Gunst SJ (1986) Effect of length history on contractile of canine tracheal smooth muscle. Am J Physiol 250:C146–C154
Kosterina N, Westerblad H, Eriksson A (2012) History effect and timing of force production introduced in a skeletal muscle model. Biomech Model Mechanobiol 11:947–957
Andersson K, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986
Schappacher-Tilp G, Leonard T, Desch G, Herzog W (2015) A novel three-filament model of force generation in eccentric contraction of skeletal muscles. PLoS ONE 10:1–16
Heidlauf T, Klotz T, Rode C, Altan E, Bleiler C, Siebert T, Röhrle O (2016) A multi-scale continuum model of skeletal muscle mechanics predicting force enhancement based on actin–titin interaction. Biomech Model Mechanobiol. doi:10.1007/s10237-016-0772-7
Nishikawa KC, Monroy JA, Uyeno TE, Yeo SH, Pai DK, Lindstedt SL (2012) Is titin a ‘winding filament’? A new twist on muscle contraction. Proc Biol Sci 279:981–990
Herrera AM, McParland BE, Bienkowska A, Tait R, Paré PD, Seow CY (2005) Sarcomeres of smooth muscle: functional characteristics and ultrastructural evidence. J Cell Sci 118:2381–2392
Van Mastrigt R, Glerum JJ (1985) Electrical stimulation of smooth muscle. J Biomed 7:2–8
Siebert T, Leichsenring K, Rode C et al (2015) Three-dimensional muscle architecture and comprehensive dynamic properties of rabbit gastrocnemius, plantaris and soleus: input for simulation studies. PLoS ONE 10:e0130985
Till O, Siebert T, Rode C, Blickhan R (2008) Characterization of isovelocity extension of activated muscle: a Hill-type model for eccentric contractions and a method for parameter determination. J Theor Biol 255:176–187
Pel JJM, Van Asselt E, Van Mastrigt R (2005) Contractile properties of inner and outer smooth muscle bundles from pig urinary detrusor. Urol Res 33:23–30
Longhurst PA, Kang J, Wein AJ, Levin RM (1990) Comparative length–tension relationship of urinary bladder strips from hamsters, rats, guinea-pigs, rabbits and cats. Comp Biochem Physiol 96A:221–225
Guilford WH, Dupuis DE, Kennedy G, Wu J, Patlak JB, Warshaw DM (1997) Smooth muscle and skeletal muscle myosins produce similar unitary forces and displacements in the laser trap. J Biophys 72:1006–1021
Ranatunga KW (1984) The force–velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol 351:517–529
Siebert T, Rode C, Herzog W, Till O, Blickhan R (2008) Nonlinearities make a difference: comparison of two common Hill-type models with real muscle. Biol Cybern 98:133–143
Böl M, Leichsenring K, Weichert C et al (2013) Three-dimensional surface geometries of the rabbit soleus muscle during contraction: input for biomechanical modelling and its validation. Biomech Model Mechanobiol 12:1205–1220
Minekus J, van Mastrigt R (2001) Length dependence of the contractility of pig detrusor smooth muscle fibres. Urol Res 29:126–133
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Pysiol 184:170–192
Ramsey RW, Street SF (1940) The isometric length–tension diagram of isolated skeletal muscle fibers of the frog. J Cell Comp Physiol 15:11–34
Schoenberg M, Podolsky RJ (1972) Length–force relation of calcium activated muscle fibers. Science 176:52–54
Gabella G, Uvelius B (1990) Urinary bladder of rat: fine structure of normal and hypertrophic musculature. Cell Tissue Res 262:67–79
Herzog W, Kamal S, Clarke HD (1992) Myofilament lengths of cat skeletal muscle: theoretical considerations and functional implications. J Biomech 25:945–948
Mow VC, Ateshian GA, Spilker RL (1993) Biomechanics of diarthrodial joints: a review of twenty years of progress. J Biomech Eng 115:460–467
Pinniger GJ, Ranatunga KW, Offer GW (2006) Crossbridge and non-crossbridge contributions to tension in lengthening rat muscle: force-induced reversal of the power stroke. J Physiol 573:627–643
Sugi H, Tsuchiya T (1988) Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J Physiol 407:215–229
Edman KA (1978) Maximum velocity of shortening in relation to sarcomere length and degree of activation of frog muscle fibres. J Physiol 278:9–10
Kosterina N, Westerblad H, Lannergren J, Eriksson A (2008) Muscular force production after concentric contraction. J Biomech 41:2422–2429
Edman KA (1975) Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol 246:255–275
Herzog W, Leonard TR (1997) Depression of cat soleus forces following isokinetic shortening. J Biomech 30:865–872
Noble MI (1992) Enhancement of mechanical performance of striated muscle by stretch during contraction. Exp Physiol 77:539–552
Linke WA, Ivemeyer M, Olivieri N, Kolmerer B, Ruegg JC, Labeit S (1966) Towards a molecular understanding of the elasticity of titin. J Mol Biol 261:62–71
Cornachione AS, Rassier DE (2012) A non-cross-bridge, static tension is present in permeabilized skeletal muscle fibers after active force inhibition or actin extraction. Cell Physiol 302:C566–C574
Corr DT, Herzog W (2016) A cross-bridge based model of force depression: Can a single modification address both transient and steady-state behavior? J Biomech 5:726–734
Arner A, Malmqvist U (1998) Cross-bridge cycling in smooth muscle: a short review. Acta Physiol Scand 164:363–372
Maruyama K, Kimura S, Tawara H (1977) Connectin, an elastic protein. J Biochem 86:339–345
Kim K, Keller TCS (2002) Smitin, a novel smooth muscle titin-like protein, interacts with myosin filaments in vivo and in vitro. J Cell Biol 156:101–111
Powers K, Schappacher-Tilp G, Jinha A, Leonard T, Nishikawa K, Herzog W (2014) Titin force is enhanced in actively stretched skeletal muscle. J Exp Biol 217:3629–3636
Bianco P, Nagy A, Kengyel A, Szatmári D, Mártonvalfi Z, Huber T, Kellermayer MSZ (2007) Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. J Biophys 93:2102–2109
Kellermayer MS, Granzier HL (1996) Calcium-dependent inhibition of in vitro thin-filament motility by native titin. FEBS Lett 380:281–286
Linke WA, Krüger M (2010) The giant protein titin as an integrator of myocyte signaling pathways. Physiology (Bethesda) 25:186–198
Joumaa V, Power GA, Hisey B, Caicedo A, Stutz J, Herzog W (2015) Effects of fiber type on force depression after active shortening in skeletal muscle. J Biomech 48:1687–1692
Yang PJ, Pham JC, Choo J, Hu JC (2013) Law of urination: all mammals empty their bladders over the same duration. J Exp Biol 404:4–5
Levin RM, Reed TP, Whitbeck C, Chichester P, Damaser M (2005) Effect of strip length on the contractile dysfunction of bladder smooth muscle after partial outlet obstruction. Urol 66:659–664
Schüssler B, Laycock J, Norton P, Stanton S (1994) Pelvic floor re-education. Principles and practice. Springer, London, pp 25–26
Schmitz A, Böl M (2011) On a phenomenological model for active smooth muscle contraction. J Biomech 44:2090–2095
Böl M, Schmitz A, Nowak G, Siebert T (2012) A three-dimensional chemo-mechanical continuum model for smooth muscle contraction. J Mech Behav Biomed Mater 13:215–229
Böl M, Schmitz A (2013) A coupled chemomechanical model for smooth muscle contraction. In: Holzapfel G, Kuhl E (eds) Computer models in biomechanics. From nano to macro. Springer, Dordrecht, pp 63–75
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG) under Grants BO 3091/9-1 and SI 841/9-1.
Author’s contribution
TS and MB conceived and designed the experiments. RM performed the experiments, analysed the data and contributed reagents/materials/analysis tools. RM, MB, and TS wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest or future conflicts regarding this article.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Menzel, R., Böl, M. & Siebert, T. Importance of contraction history on muscle force of porcine urinary bladder smooth muscle. Int Urol Nephrol 49, 205–214 (2017). https://doi.org/10.1007/s11255-016-1482-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1482-x