Abstract
Background
Renal cell carcinoma (RCC) represents the most common malignant epithelial neoplasm of the kidney. Accurate assessment of the renal masses, defining the histologic subtype and the grade of differentiation of the tumor, is vital to ensure an adequate case management as well as for staging and prognosis. Recently, diffusion-weighted imaging (DWI) magnetic resonance imaging (MRI) tends to be increasingly appealing for the clinicians as an imaging procedure of choice for the diagnosis and staging of the RCC, which is predetermined by several advantages over CT. The goal of the survey was to assess the applicability of the apparent diffusion coefficient (ADC) of the DWI MRI for the differential diagnostics, histologic subtyping, and defining the grade of differentiation of the RCC.
Methods
The study enrolled 288 adult patients with renal lesions: 188 patients with solid RCC—126 patients with clear cell subtype (ccRCC), 32 patients with papillary RCC (pRCC), 30 patients with chromophobe RCC (chRCC); 27 patient with cystic form or RCC (Bosniak cyst, category IV); 32 patients with renal angiomyolipoma (AML); 25 patients with renal oncocytoma (OC); and 16 patients with the renal abscess (AB). In total, 245 lesions were pathologically verified. As a reference, 19 healthy volunteers were included into the study. All patients underwent MRI of the kidneys, involving DWI with subsequent evaluation of the ADC.
Results
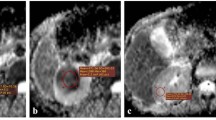
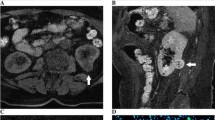
There was a reliable difference (p < 0.05) in mean ADC values between the normal renal parenchyma (NRP), solid RCC of different histologic subtypes and grades, cystic RCC, and benign renal lesions. The mean ADC values obtained in the result of the study were (×10−3 mm2/s): 2.47 ± 0.12 in NRP, 1.63 ± 0.29 in all solid RCCs, 1.82 ± 0.22 in solid ccRCC (1.92 ± 0.11—Fuhrman grade I, 1.84 ± 0.14—Fuhrman grade II, 1.79 ± 0.10—Fuhrman grade III, 1.72 ± 0.06—Fuhrman grade IV), 1.61 ± 0.07 in pRCC, 1.46 ± 0.09 in chRCC, 2.68 ± 0.11 in cystic RCC, 2.13 ± 0.08 in AML, 2.26 ± 0.06 in OC, and 3.30 ± 0.07 in AB.
Conclusion
The data received in our study demonstrate a substantial restriction of diffusion of hydrogen molecules in tissues of ccRCC in comparison with the healthy renal parenchyma preconditioned by the greater density of tumor. A statistically significant difference in mean ADC values of ccRCC with different grades of nuclear pleomorphism by Fuhrman was observed: Low-grade tumors showed higher mean ADC values compared to high-grade tumors. The modality of the MRI DWI along with ADC measurement allows to reliably differentiate between the solid RCC of main histologic subtypes and grades, cystic RCC, and the benign renal lesions.



Similar content being viewed by others
Abbreviations
- AB:
-
Renal abscess
- ADC:
-
Apparent diffusion coefficient
- AML:
-
Angiomyolipoma
- ccRCC:
-
Clear cell renal cell carcinoma
- chRCC:
-
Chromophobe renal cell carcinoma
- CT:
-
Computed tomography
- DWI:
-
Diffusion-weighted images
- FIESTA FAT SAT:
-
Fast imaging employing steady-state acquisition with fat saturation
- FRFSE:
-
Fast-recovery fast spin-echo
- FSPGR-DE:
-
Fast spoiled gradient-recalled echo dual-echo
- LAVA:
-
Liver acquisition with volume acquisition
- MRI:
-
Magnetic resonance imaging
- NRP:
-
Normal renal parenchyma
- OC:
-
Oncocytoma
- pRCC:
-
Papillary renal cell carcinoma
- RCC:
-
Renal cell carcinoma
- RCC:
-
Renal cell carcinoma
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
- SSFSE:
-
Single-shot fast spin-echo
- TE:
-
Echo time
- TR:
-
Repetition time
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27:612–624
Miguel V, Fernando L, Carlos M et al (2009) Nuclear grade prediction of renal cell carcinoma using contrasted computed tomography. J Urol 181:249
Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS (2002) Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 178:1499–1506
Sheir KZ, El-Azab M, Mosbah A, El-Baz M, Shaaban AA (2005) Differentiation of renal cell carcinoma subtypes by multislice computerized tomography. J Urol 174:451–455
Moinzadeh A, Gill IS, Finelli A, Kaouk J, Desai M (2006) Laparoscopic partial nephrectomy: 3-year followup. J Urol 175:459–462
Le Bihan D (1991) Molecular diffusion nuclear magnetic resonance imaging. Magn Reson 7:1–30
Pedrosa I, Sun MR, Spencer M, Genega EM, Olumi AF, Dewolf WC, Rofsky NM (2008) MR imaging of renal masses: correlation with findings at surgery and pathologic analysis. Radiographics 28:985–1003
Taouli B, Thakur RK, Mannelli L, Babb JS, Kim S, Hecht EM, Lee VS, Israel GM (2009) Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology 251:398–407
Sandrasegaran K, Sundaram CP, Ramaswamy R, Akisik FM, Rydberg MP, Lin C, Aisen AM (2010) Usefulness of diffusion-weighted imaging in the evaluation of renal masses. AJR Am J Roentgenol 194:438–445
Kanal E (2016) Gadolinium based contrast agents (GBCA): safety overview after 3 decades of clinical experience. Magn Reson Imaging. doi:10.1016/j.mri.2016.08.017
McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, Williamson EE, Kallmes DF (2016) Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology 278:306
Wang H, Cheng L, Zhang X, Wang D, Guo A, Gao Y, Ye H (2010) Renal cell carcinoma: diffusion-weighted mr imaging for subtype differentiation at 3.0 T. Radiology 257:135–143
Razek AA, Farouk A, Mousa A, Nabil N (2011) Role of diffusion-weighted magnetic resonance imaging in characterization of renal tumors. J Comput Assist Tomogr 35:332–336
Sun M, Lughezzani G, Jeldres C, Isbarn H, Shariat SF, Arjane P, Widmer H, Pharand D, Latour M, Perrotte P, Patard JJ, Karakiewicz PI (2009) A proposal for reclassification of the Fuhrman grading system in patients with clear cell renal cell carcinoma. Eur Urol 56:775–781
Hong SK, Jeong CW, Park JH, Kim HS, Kwak C, Choe G, Kim HH, Lee SE (2011) Application of simplified Fuhrman grading system in clear-cell renal cell carcinoma. BJU Int 107:409–415
Rosenkrantz AB, Niver BE, Fitzgerald EF, Babb JS, Chandarana H, Melamed J (2010) Utility of the apparent diffusion coefficient for distinguishing clear cell renal cell carcinoma of low and high nuclear grade. AJR Am J Roentgenol 195:W344–W351
Mytsyk Y, Borys Y, Komnatska I, Dutka I, Shatynska-Mytsyk I (2014) Value of the diffusion-weighted MRI in the differential diagnostics of malignant and benign kidney neoplasms—our clinical experience. Pol J Radiol 79:290–295
Lassel EA, Rao R, Schwenke C, Schoenberg SO, Michaely HJ (2014) Diffusion-weighted imaging of focal renal lesions: a meta-analysis. Eur Radiol 24:241–249
Zhang H, Gan Q, Wu Y, Liu R, Liu X, Huang Z, Yuan F, Kuang M, Song B (2016) Diagnostic performance of diffusion-weighted magnetic resonance imaging in differentiating human renal lesions (benignity or malignancy): a meta-analysis. Abdom Radiol (NY) 41:1997–2010
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mytsyk, Y., Dutka, I., Borys, Y. et al. Renal cell carcinoma: applicability of the apparent coefficient of the diffusion-weighted estimated by MRI for improving their differential diagnosis, histologic subtyping, and differentiation grade. Int Urol Nephrol 49, 215–224 (2017). https://doi.org/10.1007/s11255-016-1460-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1460-3