Abstract
Purpose
To assess the impact on safety and efficiency of onabotulinumtoxinA (BOTOX1, Allergan, Inc.) treatment in patients with an overactive bladder.
Materials and Methods
We searched the PubMed®, Embase®, and Cochrane Library Databases to identify all randomized controlled trials comparing the outcomes of onabotulinumtoxinA and placebo for overactive bladder. The outcomes included reductions in overactive bladder symptoms or improvements in the function of bladder and the side effects of two treatments. The Cochrane Collaboration Review Manager software (RevMan 5.1.4) was used for statistical analysis.
Results
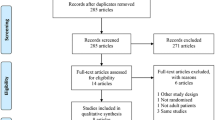
The study inclusion criteria were met by eight randomized controlled trials involving 1875 patients. The synthesized data from these randomized controlled trials indicated that onabotulinumtoxinA was better than placebo in decreasing most overactive bladder symptoms (p < 0.00001, p < 0.00001, p < 0.00001, p < 0.00001, p = 0.0003) in the micturition, urgency, urinary incontinence, urgency urinary incontinence (UUI), and nocturia per day change, respectively; however, the maximum cystometric capacity change from the baseline appeared not to be significantly different between two methods (p = 0.05). In addition, the side effects in the onabotulinumtoxinA group were more serious than the placebo group (p < 0.00001, p = 0.009, p = 0.07, p < 0.0001, p = 0.03 in the UTI, bacteriuria, dysuria, urinary retention, residual urine volume, respectively).
Conclusions
Compared with the placebo, onabotulinumtoxinA had significantly and clinically relevant reductions in overactive bladder symptoms, but it also leaded to more side effects.





Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49
Abrams P, Artibani W, Cardozo L et al (2009) Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn 28:297
Chapple C (2000) Muscarinic receptor antagonist in the treatment of overactive bladder. Urology 55:33–46
Gormley EA, Lightner DJ, Faraday M et al (2012) Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol 188:2455
Schurch B, Stöhrer M, Kramer G et al (2000) Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 164:592
Apostolidis A, Dasgupta P, Denys P et al (2009) Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol 55:100–119
Karsenty G, Denys P, Amarenco G et al (2008) Botulinum toxin A (Botox) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic literature review. Eur Urol 53:275–287
Dong M, Yeh F, Tepp WH et al (2006) SV2 is the protein receptor for botulinum neurotoxin A. Science 312:592–596
Simpson L (1980) Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther 1980(212):16–21
Apostolidis A, Popat R, Yiangou Y et al (2005) Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intra-detrusor injections of botulinum toxin for human detrusor overactivity. J Urol 174:977–982
Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.4 (updated March 2011). The Cochrane collaboration 2011. http://www.cochrane-handbook.org Accessed 27 Feb 2012
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. BMJ 339:b2535
Chapple C, Sievert KD, MacDiarmid S et al (2013) OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 64:249–256
Chuang YC, Kaufmann JH, Chancellor DD et al (2014) Bladder instillation of liposome encapsulated onabotulinumtoxinA improves overactive bladder symptoms: a prospective, multicenter, double-blind, randomized trial. J Urol 192:1743–1749
Denys P, Le Normand L, Ghout I et al (2012) Efficacy and safety of low doses of onabotulinumtoxinA for the treatment of refractory idiopathic overactive bladder: a multicentre, double-blind, randomised, placebo-controlled dose-ranging study. Eur Urol 61:520–529
Dmochowski R, Chapple C, Nitti VW et al (2010) Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 184:2416–2422
Nitti VW, Dmochowski R, Herschorn S et al (2013) OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 189:2186–2193
Rovner E, Kennelly M, Schulte-Baukloh H et al (2011) Urodynamic results and clinical outcomes with intradetrusor injections of onabotulinumtoxinA in a randomized, placebo-controlled dose-finding study in idiopathic overactive bladder. Neurourol Urodyn 30:556–562
Tincello DG, Kenyon S, Abrams KR et al (2012) Botulinum toxin a versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX study). Eur Urol 62:507–514
Stewart WF, Van Rooyen JB, Cundiff GW et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20:327–336
Chapple CR, Khullar V, Gabriel Z et al (2008) The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54:543–562
Yu YF, Nichol MB, Yu AP et al (2005) Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California medicaid program. Value Health 8:495–505
Apostolidis A, Dasgupta P, Fowler CJ (2006) Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 49:644–650
Sahai A, Khan M, Dasgupta P (2007) Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol 177:2231–2236
Chuang YC, Tyagi P, Huang CC et al (2009) Urodynamic and immunohistochemical evaluation of intra- vesical botulinum toxin A delivery using liposomes. J Urol 182:786
Yokoyama T, Chancellor MB, Oguma K et al (2012) Botulinum toxin type A for the treatment of lower urinary tract disorders. Int J Urol 19:202–215
Acknowledgments
This study was supported by the Prostate Cancer Foundation Young Investigator Award 2013, the National Natural Science Foundation of China (Grant No. 81300627), National Science Foundation for Young Scholars of China (Grant No. 81300579), and Pillar Program from Science and Technology Department of Sichuan Province (Grant No. 2014JY0219).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Yi Sun and Deyi Luo have contributed equally to this work.
Yi Sun and Deyi Luo share the co-first author.
Rights and permissions
About this article
Cite this article
Sun, Y., Luo, D., Tang, C. et al. The safety and efficiency of onabotulinumtoxinA for the treatment of overactive bladder: a systematic review and meta-analysis. Int Urol Nephrol 47, 1779–1788 (2015). https://doi.org/10.1007/s11255-015-1125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1125-7