Abstract
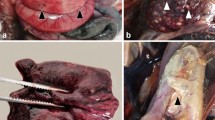
In the summers of 2018 and 2019, a disease outbreak stroke 25 broiler chicken farms and 3 broiler breeder farms in different Governorates in Egypt. The disease caused a mortality rate ranging from 3.2 to 9%. Postmortem examination showed petechial hemorrhage in the breast and thigh muscles, thymus gland, and peritoneal cavity and extensive hemorrhages in the kidneys. A total of 140 liver, kidney, lung, skeletal muscles, thymus, and spleen samples were collected. Twenty-eight pooled samples were created and examined by PCR and histopathological examination to identify the causative pathogens. All collected samples were PCR-negative to Newcastle disease virus (NDV), avian influenza viruses (H5, H9, and H7), infectious bursal disease virus (IBDV), infectious bronchitis virus (IBV), and fowl adenovirus (FadV). Leucocytozoon caulleryi (L. caulleryi) genetic material was identified by PCR in 17 out of the 28 collected samples (61%). Five chicken farms (18%) showed positive PCR results for both L. caulleryi and chicken anemia virus (CAV). Histopathological examination revealed unilocular megaloschizonts in thymus, skeletal muscle, and lung as well as massive hemorrhages in parenchymatous organs. Nucleotide sequences of the identified pathogens were compared with other reference sequences available in the GenBank. The identified L. caulleryi has a close relationship with those previously detected in Asia, indicating potential transmission route of the parasite. The CAV has a close genetic relation with CAVs previously identified in Egypt. Furthermore, a real-time PCR for rapid, specific, and quasiquantitative detection of L. caulleryi was developed with a detection limit of 100 genome copies per reaction.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Atkinson, C.T. and Van-Riper, C., 1991. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon and Haemoproteus. In: Loye, J.E. and Zuk, M. (eds). Bird-parasite interactions: ecology, evolution and behaviour. University of Oxford Press, Oxford, pp., 19–48.
Capua, I. and Terregino, C., 2009. Clinical traits and pathology of avian influenza infections, guidelines for farm visit and differential diagnosis. In: Avian Infuenza and Newcastle disease, A Field and Laboratory Manual. Capua, I. and Alexander, D.J., eds. Springr, pp., 45–72.
Chaharaein, B., Omar, A.R., Aini, I., Yusoff, K. and Hassan S.S., 2009. Detection of H5, H7 and H9 subtypes of avian influenza viruses by multiplex reverse transcription-polymerase chain reaction. Microbiological research, 164, 174-179.
Creelan, J.L., Graham, D.A. McCullough, S.J., 2002. Detection and differentiation of pathogenicity of avian paramyxovirus serotyp and e 1 from field cases using one-step reverse transcriptase polymerase chain reaction. Avian Pathology, 31(5), 493-499.
Cumming, R.B., 1963. Infectious avian nephrosis (uraemia) in Australia. Australian Veterinary Journal 39, 145-147.
Deviche, P., Greiner, E.C. and Manteca X., 2001. Seasonal and age related changes in blood parasite prevalence in dark-eyed Juncos (Junco hyemalis, Aves, Passeriformes). Journal of Experimental Zoology, 289, 456-466.
El-Tholoth, M. and Abou El-Azm, K.I., 2019. Molecular detection and characterization of fowl adenovirus associated with inclusion body hepatitis from broiler chickens in Egypt. Tropical Anim Health and Production, 51(5),1065-1071.
Goto, M., Fujihara, H. and Morita M., 1996. Pathological studies of leucocytozoonosis in chickens. The Japanese Journal of Veterinary Science, 28, 183-190.
Hellgren, O., Waldenström, J. and Bensch, S., 2004. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood[J]. Journal of Parasitology, 90(4), 797–802.
Lee. H.R., Koo, B.S., Jeon, E.O., Han, M.S., Min, K.Ch., Lee, S.B., Bae, Y. Mo, I.P., 2016. Pathology and molecular characterization of recent Leucocytozoon caulleryi ca and ses in layer flocks. The Journal of Biomedical Research, 30(6), 517-524.
Levine, N. D., Corliss, N. D., Cox, F. E. G., Deroux, J., Grain, J. Honigberg, B.M., Leedale, G.F., Loeblich III, A. R., Lorn, J., Lynn, J., Merinfeld, E. G., Page, F.C., Poljansky, G., Sprague, V., Vavra, J. and Wallac, F.G., 1980. A newly revised classification of the protozoa. Journal of Protozoology, 27, 37-58.
Lucio, B., Schat K.A. and Shivaprasad H.L., 1990. Identification of the chicken anemia agent, reproduction of the disease, and serological survey in the United States. Avian Diseases, 34, 146-153.
McDougald, L., 2013. Protozoal Infections. In: Swayne, D., Glisson, J., McDougald, L., Nolan, L., Suarez, D., Nair, V., eds. Diseases of Poultry. 13 ed. Wiley-Blackwell, Ames, Iowa, PP., 1147–1201.
Milhous, W. and Solis, J., 1973. Turkey leucocytozoon infection. 3. Ultrastructure of Leucocytozoon smithi gametocytes. Poultry Science, 52, 2138-46.
Miura, S., Ohshima, K., Itakura, C. and Yamogiwa S., 1973. A histopathological study on Leucocytozoonosis in young hens. The Japanese Journal of Veterinary Science, 35, 175-181.
Morii, T., 1992. A Review of Leucocytozoon Caulleryi infection in chickens. Journal of protozoology research, 2, 128-133.
Murdock, C.C, Foufopoulos, J. and Simon, C.P., 2013. A transmission model for the ecology of an avian blood parasite in a temperate ecosystem. PLos ONE, 8(9), e76126.
OIE., 2008. Infectious bursal disease (Gumboro disease). OIE Terrestrial manual. Chapter 2.3.12, 549–565.
Omori, S., Sato, Y., Hirakawa, S., Isobe, T., Yukawa, M. and Murata, K., 2008. Two extra chromosomal genomes of Leucocytozoon caulleryi: Complete nucleotide sequences of the mitochondrial genome and existence of the apicoplast genome. Parasitology Research, 103, 953-957.
Pope, C.R., 1991. Chicken anemia agent. Veterinary Immunology and Immunopathology, 30, 51-65.
Pringle, C.R., 1999. Virus taxonomy at the XIth International congress of virology, Sydney, Australia, 1999. Archives of Virology, 144, 2065-2069.
Rosario, K., Breitbart, M., Harrach, B., Segales, J., Delwart, E., Biagini, P. and Varsani, A., 2017. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Archives of Virology, 162, 1447–1463.
Rintamaki, P.T., Ojanen, W., Pakkala, H. and Tynjala M., 1998. Blood parasites of migrating willow warblers (Phylloscopus trochillus) at a stopover site. Canadian Journal of Zoology, 76, 984-988
Slomka, M,J., Coward, V.J., Banks, J., Londot, B.Z., Brown, I.H., Voermans, J., Koch, G., Handberg, K.J., Jorgensen, P.H., Cherbonnel-Pansart, M., Jestin, V., Cattoli, G., Capua, I., Ejdersund, A., Thoren, P. and Czifa, G., 2007. Identification of sensitive and specific avian influenza polymerase chain reaction methods through blind ring trials organized in the Euopean Union. Avian Diseases, 51, 227-234.
Suprihati, E. and Yuniarti W.M., 2017. The phylogenetics of Leucocytozoon caulleryi infected broiler chickens in endemic areas in Indonesia. Veterinary World, 10(11), 1324-1328.
Taylor, S.P., 1989. National Veterinary Services Laboratory (NVSL), Ames, IA: USDA, ARS; 1989. Cited by Hermann, J., Koski, D., Taylor, S. and Gatewood, D., 2012. Evaluation on the analytical sensitivity of a polymerase chain reaction assay for the detection of chicken anemia virus in avian vaccines. Biologicals, 40, 266-269.
Terregino, C. and Capua I., 2009. Clinical traits and pathology of Newcastle diseases infections, guidelines for farm visit and differential diagnosis. In: Avian Infuenza and Newcastle disease, A Field and Laboratory Manual. Capua, I., Alexander, D.J., eds. Springr, , chapter 9, pp., 113–122.
Valkiunas, G., 2005. Avian malaria parasites and other Haemosporidia. CRC Press, Boca Raton, Florida, USA.
WHO, Geneva., 2005. Recommended laboratory tests to identify avian influenza A virus in specimens from humans. 2005, 1–7.
Williams, A.K., Wang, L., Sneed, L.W. and Collisson E.W., 1992. Comparative analysis of the nucleocapsid genes of several strains of infectious bronchitis virus and other coronaviruses. Virus Research, 25 (3), 213-222
Zeryehun, T., Bejo, M.H. and Rasedee A., 2012. Hemorrhagic and clotting abnormalities in infectious bursal disease in specific-pathogen-free chicks. World Applied Sciences Journal, 16 (98), 1123-1130.
Zhao, W., Liu, J., Xu, R., Zhang, C., Pang, Q., Chen, X., Liu, S., Hong, L., Yuan, J., Li, X., Chen, Y., Li, J. and Su, Z., 2015. The gametocytes of Leucocytozoon sabrazesi infect chicken thrombocytes, not other blood cells. PLoS One, 10(7), e0133478.
Author information
Authors and Affiliations
Contributions
KIA-E, designed the study, methodology, analyzed the data, and wrote the paper. MFH, contributed to pathological examination of the collected samples and data analysis. AM, contributed to methodology and analyzed the data. TR, GNG, and SAE, contributed to molecular characterization of the collected samples. HHB, provided critical feedback on the manuscript. MET, designed the study, methodology, analyzed the data, wrote the paper, and supervised the research project. All authors revised the final manuscript before submission.
Corresponding author
Ethics declarations
Ethics approval
Collection of samples from chickens were done according to the ethical guidelines of University Mansoura and The ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. All experiments were conducted in compliance with the instructions stipulated by Ethical Committee for Medical Research at Faculty of Veterinary Medicine, Mansoura University, Egypt and in accordance with local laws and regulations.
Consent to participate
Chicken farm owners were aware of the objectives of this work and gave oral consent in harmony with the national ethical regulations.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
EL-Azm, K.I.A., Hamed, M.F., Matter, A. et al. Molecular and pathological characterization of natural co-infection of poultry farms with the recently emerged Leucocytozoon caulleryi and chicken anemia virus in Egypt. Trop Anim Health Prod 54, 91 (2022). https://doi.org/10.1007/s11250-022-03097-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03097-8