Abstract
While many studies investigate animal-related risk factors for disease, few have considered environmental or spatial risk factors in the dynamics of bovine tuberculosis (bTB) and brucellosis. In the Ruaha ecosystem of Tanzania, we investigated the role of household location as a predictor for infection with Mycobacterium bovis and exposure to Brucella in pastoralist and agropastoralist cattle herds in a typical African wildlife-livestock-human interface. ArcGIS was utilized to calculate Euclidian distances between households and the nearest river, village center, protected area, and other infected households, followed by multivariate logistic regression to assess the association between risk factors and herd-level bTB and Brucella outcomes. Global and local spatial clustering of bTB-infected and Brucella-exposed herds was explored using the Cuzick-Edward’s test and SaTScan spatial scan statistics. Households located farther from rivers and closer to village centers and herds belonging to agropastoralists were more likely to have bTB-positive cattle. Risk of Brucella exposure increased with proximity to protected areas. One spatial cluster of households with Brucella spp. seropositive cattle was identified. Spatial factors may be useful for assessing disease risk and for formulating intervention and control strategies for households that manage cattle in ecosystems characterized by seasonally limited resources and intense wildlife-livestock interfaces.
Similar content being viewed by others
Introduction
Wildlife-livestock-human interfaces can be found in proximity to many unfenced protected areas in Africa. Growing human and animal populations, and changes in the availability of water and grazing, may intensify competition for resources and bring wildlife, livestock, and people closer together, which can increase the risk of disease transmission among populations. The southern border of the Greater Ruaha ecosystem in Tanzania is an example of a location with an intense interface, where the animal and human populations are forced to share dwindling water and other resources, especially in the dry season, and where the water scarcity has been amplified due to climate change and unsustainable upstream irrigation practices (Coppolillo et al. 2008; Mwakalila 2011). Zoonotic diseases such as bovine tuberculosis (bTB) and brucellosis are especially of concern in this area, and surveys conducted by the Health for Animal and Livelihood Improvement (HALI) project (Mazet et al. 2009) in regional pastoralist and agropastoralist households detected high cattle herd prevalences of bTB (18 %) and Brucella (42 %). Bovine tuberculosis and exposure to Brucella spp. were also confirmed in regional wildlife, and identical Mycobacterium bovis strains were isolated from wildlife and cattle, supporting concerns about interspecies sharing of pathogens (Clifford et al. 2013).
Pastoralists and agropastoralists are considered high-risk groups for contracting bTB and brucellosis due to their close contact with livestock and diet rich in animal products (Mfinanga et al. 2003). Risk factors for bTB infection and exposure to Brucella in cattle include herd size, age, stocking density, and production system (Cleaveland et al. 2007; Swai and Schoonman 2010, 2012). Household location may influence access to water and grazing land, determine livestock management practices, and affect the degree of interaction with other cattle herds and wildlife. Coppolillo et al. (2009) found household location to be a risk factor for loss of livestock due to acute disease in the Ruaha area and that households located at greater distance from village centers and surface water reported more livestock losses. Previous local livestock surveys identified behavioral risk factors for livestock bTB infection at the household level (HALI project, unpublished), but environmental and locational risk factors that could influence the occurrence of bTB and Brucella infection in cattle herds have not been evaluated. Knowing whether the risk of bTB infection or exposure to Brucella spp. differs spatially is of importance for understanding infection risks and planning possible veterinary and public health interventions and is of value to pastoralists who can make more informed choices on how to manage disease risks in their animals. The goal of this study was to investigate spatial risk factors and clustering for bTB herd infection and exposure to Brucella spp. in livestock herds belonging to pastoralists and agropastoralists in this typical wildlife, livestock, and human interface area. The information from this study may be relevant in other ecosystems with seasonally limited resources and intense wildlife-livestock interfaces.
Material and methods
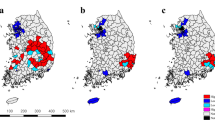
The HALI project conducted livestock health surveys in pastoralist households of the Pawaga and Idodi Divisions in the Iringa district of Tanzania from 2007 to 2009. The Idodi and Pawaga Divisions (between −7.317 to −7.600 latitude and 35.083 to 35.483 longitude) border the Pawaga-Idodi Wildlife Management Area and Lunda Game Controlled Area south and east of Ruaha National Park (Fig. 1). The area is semiarid and predominantly populated by Maasai and Barabaig pastoralists, Sukuma agropastoralists, and subsistence agriculturalists in the villages of approximately 20–200 households. It is an intense interface between wildlife and livestock, as the park boundaries are unfenced. Major sources of surface water are the Great and Little Ruaha Rivers, and the Great Ruaha River dries and floods seasonally each year. Each of the households was visited by HALI team members, and a questionnaire about herd demographics and livestock management practices was administered in Swahili. Cattle were tested for bTB by the Single Comparative Intradermal Tuberculin Test (SCITT) and in a subset of households also for exposure to Brucella spp. by the Rose Bengal plate test (RBPT). Studies have found the SCITT test to have a sensitivity of 52–100 % and specificity of 75.6–99 % (De la Rua-Domenech et al. 2006; Alvarez et al. 2012) and the RBPT to have a sensitivity and specificity of 87 and 97.8 %, respectively (Godfroid et al. 2010). The locations of each household and village center were measured by handheld GPS (Garmin Rino GPS) using the WGS1984 coordinate system. The household location was considered interchangeable with herd location since bomas were located in close proximity to the household. All research activities in Tanzania were approved by the Tanzania Commission on Science and Technology and the University of California Davis Institutional Animal Care and Use Committee (Protocol #12394). All participating household consented to enroll in the study.
The bTB and brucellosis test results, location of household (latitude and longitude), data on herd size, ethnic group, village, occurrence of chronic disease, and whether the herds were grazed beyond village borders were utilized for this analysis. The relevant survey questions are provided in the Appendix. Household and village locations were mapped using ArcGIS (v. 10.1, ESRI, Redlands, CA). Distances in kilometers between the household and the nearest village center, protected area boundary, river, and infected household were calculated using the “Near tool” in ArcGIS.
Descriptive statistics was used to evaluate the distribution of distances from the households to the nearest river, protected area, village center, bTB-infected herd, and Brucella spp. seropositive herds. The proportion of households with bTB reactors and Brucella spp. seropositive cattle was described by ethnic group association, mixing with other herds, herd size, chronic disease occurrence in the herd, and grazing beyond village borders, and for bTB-infected herds, also Brucella spp. seropositive herd status.
A mixed effects multivariate logistic regression model, which included all distance parameters as main predictors and a random effect for “village,” was built using forward selection, with individual confounders retained using the Wald’s and likelihood ratio tests and P values ≤0.10. All descriptive statistics and regression analyses were performed using Stata (v. IC 12.1, StataCorp, College Station, TX).
Global spatial clustering was evaluated with the Cuzick-Edward’s test (Cuzick and Edwards 1990) using an Excel spreadsheet add-in (SSTAT v.470, University of California, Davis). The analysis was performed up to a level of six nearest neighbors. Local spatial clustering was also assessed using the Bernoulli model of a spatial scan test (Kulldorf 1997) in SaTScan (9.1.1, Boston, MA). The Bernoulli model for dichotomous (0/1) case control data assumes no underlying population distribution and imposes a large number of ellipses or circles on the map at different locations with a maximum cluster size of up to 50 % of the population at risk. The significance of clusters was determined through 999 Monte Carlo, and clusters were evaluated for significance at P ≤ 0.05.
Results
Data from 97 households were utilized in this study. The farthest distance to surface water, protected area, and village center was 9.19, 12.36, and 7.77 km, respectively (Table 1). The dominant ethnic group was Maasai pastoralists (54/97), then Sukuma agropastoralists (33/97), and Barabaig pastoralists (10/97) (Table 2). A total of 16/97 (16.5 %) of herds had at least one bTB-positive reactor, and of the 88 herds tested for exposure to Brucella spp., 40.9 % had at least one seropositive animal (Table 2).
Findings from the multivariate regression analysis indicated that distance to river, village center, and ethnic group were significantly associated with bTB-positive herd status. For every kilometer that a household was farther away from the river, the household became 1.46 times more likely (P = 0.046) to have a bTB-positive cattle herd (Table 3). In addition, a household had 1.51 times higher odds of having a bTB-positive cattle herd for every kilometer it was closer to the village center (P = 0.001). A third of the Sukuma herds had bTB-positive cattle, and Sukuma households were also over four times more likely to have positive animals than Maasai households (P = 0.009). There was no association between bTB-positive herd status and distance to the protected area or to other infected herds (Table 3).
In contrast, increasing proximity to a protected area was a significant risk factor for exposure to Brucella spp., and for every kilometer that a household was located closer to the protected area border, the odds of having seropositive cattle increased by 1.36 (P = 0.001) (Table 4). Mixing with other herds (P = 0.097) was also associated with higher odds of having Brucella seropositive animals and was retained in the model to improve model fit. Only 7/88 herds had both bTB-positive and Brucella spp. seropositive animals, and infection or exposure to the other pathogen was not significant in any of the models.
Significant overall clustering was identified for Brucella seropositive herds on all levels above the first nearest neighbor (P = 0.003–0.010) with the Cuzick-Edward’s test. The spatial scan identified one significant elliptical local area cluster of nine Brucella spp. seropositive herds (P = 0.018) within a 57.61-km2 area (Fig. 1). Eight households in the cluster belonged to Sukuma agropastoralists, and one household belonged to Maasai pastoralists. The observed to expected ratio, relative risk, and log likelihood ratio were 2.44, 2.93, and 8.80, respectively. No significant clustering of bTB-positive households was identified.
Discussion
The risk for bTB herd infection or exposure to Brucella spp. appeared to vary with household location in relation to water sources, villages, or proximity to protected area. Both bTB and brucellosis have been reported in many wildlife, livestock, and human interface areas in Africa (Munyeme and Munang'andu 2011; Gomo et al. 2012; Jori et al. 2013; Katale et al. 2013), and the results of this study may therefore be relevant for other similar environments. Cattle herds belonging to households located farther away from rivers had higher odds of bTB infection, which agrees with the study on spatial risk factors for livestock loss due to acute disease conducted in the same area by Coppolillo et al. (2009). As hypothesized by Coppolillo et al., stress due to cattle having to walk longer distances and poorer water quality in locations far from surface water could negatively impact production and could increase the disease susceptibility and consequently the rate of disease. In agreement with our findings, a study of Sukuma agropastoralists in western Tanzania found that cattle traveling longer daily distances had lower milk yields, and that household location in relation to surface water influenced the daily herding distance (Coppolillo 2000). Poor water quality has been found to be a risk factor for bTB infection in Uganda and Ethiopia (Oloya et al. 2007; Gumi et al. 2011), and although we did not have information on the type of drinking water sources used by the pastoralist herds, it is plausible that households located far away from surface water made more use of temporary, stagnant water holes in the dry season. Although direct environmental transmission of bTB through shared water has not been reported, M. bovis has been detected in environmental samples in East Africa (Khera et al. submitted), and experimental studies have confirmed that M. bovis can survive for multiple days in water, even at 20 °C (Fine et al. 2011). Environmental persistence of M. bovis has been proposed to play a role in the transmission of bTB in the UK (Courtenay et al. 2006), and environmental transmission potentially could occur among African wildlife and livestock at an intense interface, such as around dwindling, stagnant water holes. A possible confounding factor that requires further investigation is that households living farthest from the water sources may have the poorest socioeconomic status, which could influence the health status of livestock. However, if herd size (categorized as in Table 2) was used as an indicator of socioeconomic status, no significant difference in the distance from the household to the river could be found using the Kruskal-Wallis test (P = 0.244).
Proximity to a village center was also a significant risk factor for having bTB-positive cattle, possibly because of more interaction with other herds in these locations. No clusters for bTB infection were detected, and distance to other bTB-infected households was nonsignificant; however, only pastoralist herds were surveyed in this study, and the higher likelihood of bTB infection near the villages could indicate that village livestock belonging to nonpastoralist households could be sources of bTB infection. Distance to protected area, as an indicator of wildlife density, did not appear to be associated with bTB-positive status, and this finding is consistent with that of studies in northern (Cleaveland et al. 2007) and central Tanzania (Mwakapuja et al. 2013). Bovine tuberculosis was confirmed in several Ruaha wildlife species including the African buffalo (Syncerus caffer) (Clifford et al. 2013), an important wildlife maintenance host for bTB in Africa (De Vos et al. 2001), but the extent to which Ruaha wildlife plays a role in the epidemiology of bTB in cattle remains unknown.
Ethnic group was also found to be a risk factor, with the Sukuma agropastoralists having the highest odds of bTB-positive cattle followed by the Maasai, whereas all the Barabaig herds were negative. Of the three ethnic groups, the Sukuma is the most sedentary and practice mixed crop farming and livestock production with limited transhumance (Homewood 2008). The Barabaig is the most nomadic of the three groups, although they sometimes maintain a permanent homestead. These findings are in contrast to other studies that have found that the degree of transhumance is a risk factor for bTB infection in cattle (Oloya et al. 2007; Munyeme et al. 2009), possibly because of increased herd mixing and larger herd size. Sukuma households were generally located farther away from rivers (median distance 4.6 km) than Maasai or Barabaig households (median distance 2.5 and 1.5 km, respectively), but interestingly, both distance and ethnic group remained significant in the regression, indicating that both location- and group-specific factors, such as differences in cattle management, influenced the occurrence of bTB in cattle herds.
Exposure to Brucella spp. was more likely in households located closer to protected areas, a finding that agrees with that of other studies from Tanzania and Zambia that found exposure to Brucella spp. to be more prevalent in wildlife-livestock interface areas (Jiwa et al. 1996; Muma et al. 2006). African wildlife can become infected with brucellosis (Godfroid 2002; Motsi et al. 2013), and in 2011, 3/19 live African buffalo from within Ruaha National Park were found seropositive for Brucella spp. with the Rose Bengal test (HALI unpublished). However, the wider prevalence of brucellosis in Ruaha wildlife is unknown; it is therefore unclear to what extent wildlife plays a role in the epidemiology of brucellosis in the pastoralist cattle.
Mixing with other herds was also identified as a possible risk factor for Brucella spp. exposure, although not highly significant. It is likely that herd mixing and contact to infected tissues mainly occurs when animals are grazed in the same areas, and mixing may be more common among neighboring households who may graze their cattle together during the daytime. We did not have any specific information on how households were mixing their cattle, but family/neighbor relationships could explain the cluster of seropositive herds found on the spatial scan, where 8/9 of the households were Sukuma agropastoralists. Other studies have found that both mixing with other herds and increasing herd size was associated with higher seroprevalences of Brucella spp. (McDermott and Arimi 2002; Matope et al. 2010) and that the seroprevalence was lowest in zero-grazing small-holder production systems with minimal contact with other cattle (McDermott and Arimi 2002).
Limitations to this study were the lack of additional confirmatory diagnostic testing, the fact that we only could calculate Euclidian (straight line) distances, and that the daily grazing ranges or seasonal migration of cattle herds could not be taken into consideration. The strength of this study was the inclusion of a high number of local pastoralist households from several ethnic groups, and although individual households of sedentary agriculturalist were not sampled, the village location can be assumed to approximate the risk stemming from this subpopulation.
In conclusion, our study showed that the risk of bTB infection and exposure to Brucella spp. in pastoralist and agropastoralist cattle may be influenced by household location in an intense wildlife-livestock-human interface setting. Assessment of additional risk factors such as herd management practices, herd demographics, socioeconomic status, annual variations in climate and feed availability, and livestock production indices, as well as access to veterinary care, are necessary to more fully understand the epidemiology of bTB and brucellosis at these interfaces. From a population heath perspective, this study shows how knowledge of spatial factors can be useful for assessing risk and can contribute to formulating intervention and control strategies that will benefit villages and households that manage cattle in this type of ecosystem.
References
Alvarez, J., Perez, A., Bezos, J., Marques, S., Grau, A., Saez, J. L., Minguez, O., de Juan, L. and Dominguez, L., 2012. Evaluation of the sensitivity and specificity of bovine tuberculosis diagnostic tests in naturally infected cattle herds using a Bayesian approach, Veterinary Microbiology, 155, 38-43
Cleaveland, S., Shaw, D.J., Mfinanga, S.G., Shirima, G., Kazwala, R.R., Eblate, E. and Sharp, M., 2007. Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations, Tuberculosis (Edinb), 87, 30-43
Clifford, D.L., Kazwala, R.R., Sadiki, H., Roug, A., Muse, E.A., Coppolillo, P.C. and Mazet, J.A.K., 2013. Tuberculosis infection in wildlife from the Ruaha ecosystem Tanzania: implications for wildlife, domestic animals, and human health, Epidemiology and Infection, doi:10.1017/S0950268813000836
Coppolillo, P., 2000. The Landscape ecology of pastoral herding: Spatial analysis of land use and livestock production in East Africa, Human Ecology, 28, 527-560
Coppolillo, P., Clifford, D. L. and Mazet, J. A. K., 2008. The unintended consequences of development assistance: The case of Usangu in Tanzania, Research Brief 08-02-HALI. Global Livestock Collaborative Research Support Program (CRSP). Available: http://glcrsp.ucdavis.edu/publications/?project=hali. Accessed February 4th, 2014.
Coppolillo, P., Clifford, D., Dickman, A., Masozera, M., Nguvava, M., Kazwala, R., Erickson, J. and Mazet, M., 2009. Landscape factors associated with livestock disease deaths in Idodi and Pawaga Divisions, Tanzania, Research Brief 09-01-HALI. Global Livestock Collaborative Research Support Program (GLCRP). Available: http://glcrsp.ucdavis.edu/publications/?project=hali. Accessed February 4th, 2014.
Courtenay, O., Reilly, L.A., Sweeney, F.P., Hibberd, V., Bryan, S., Ul-Hassan, A., Newman, C., Macdonald, D.W., Delahay, R.J., Wilson, G.J. and Wellington, E.M., 2006. Is Mycobacterium bovis in the environment important for the persistence of bovine tuberculosis? Biology Letters, 2, 460-462
Cuzick, J. and Edwards, R., 1990. Spatial clustering for inhomogeneous populations, Journal of the Royal Statistical Society, Series B, 52, 73 -104
de la Rua-Domenech, R., Goodchild, A. T., Vordermeier, H. M., Hewinson, R. G., Christiansen, K. H. and Clifton-Hadley, R. S., 2006. Ante mortem diagnosis of tuberculosis in cattle: A review of the tuberculin tests, [gamma]-interferon assay and other ancillary diagnostic techniques, Research in Veterinary Science, 81, 190-210
De Vos, V., Bengis, R.G., Kriek, N.P., Michel, A., Keet, D.F., Raath, J.P. and Huchzermeyer, H.F., 2001. The epidemiology of tuberculosis in free-ranging African buffalo (Syncerus caffer) in the Kruger National Park, South Africa, Onderstepoort Journal of Veterinary Research, 68, 119-130
Fine, A.E., Bolin, C.A., Gardiner, J.C. and Kaneene, J.B., 2011. A Study of the Persistence of Mycobacterium bovis in the environment under natural weather conditions in Michigan, USA, Veterinary Medicine International, 2011, 765430, doi:10.4061/2011/765430.
Godfroid, J., 2002. Brucellosis in wildlife, Revue Scientifique et Technique Office International des Epizooties, 21, 277-286
Godfroid, J., Nielsen, K. and Saegerman, C., 2010. Diagnosis of brucellosis in livestock and wildlife, Croatian Medical Journal, 51, 296-305
Gomo, C., de Garine-Wichatitsky, M., Caron, A. and Pfukenyi, D.M., 2012. Survey of brucellosis at the wildlife-livestock interface on the Zimbabwean side of the Great Limpopo Transfrontier Conservation Area, Tropical Animal Health and Production, 44, 77-85
Gumi, B., Schelling, E., Firdessa, R., Aseffa, A., Tschopp, R., Yamuah, L., Young, D. and Zinsstag, J., 2011. Prevalence of bovine tuberculosis in pastoral cattle herds in the Oromia region, southern Ethiopia, Tropical Animal Health and Production, 43, 1081-1087
Homewood, K. 2008. Ecology of African pastoralist societies, Ohio University Press Athens, OH, USA
Jiwa, S.F.H., Kazwala, R.R., Tungaraza, R., Kimera, S.I. and Kalaye, W.J., 1996. Bovine brucellosis serum agglutination test prevalence and breed disposition according to prevalent management systems in the Lake Victoria zone of Tanzania, Preventive Veterinary Medicine, 26, 341-346
Jori, F., Mokospasetso, M., Etter, E., Munstermann, S., Newman, S.H. and Michel, A., 2013. Preliminary assessment of bovine tuberculosis at the livestock/wildlife interface in two protected areas of northern Botswana, Transboundary and Emerging Diseases, 60, 28-36. doi:10.1111/tbed.12110.
Katale, B.Z., Mbugi, E.V., Karimuribo, E.D., Keyyu, J.D., Kendall, S., Kibiki, G.S., Godfrey-Faussett, P., Michel, A.L., Kazwala, R.R., van Helden, P. and Matee, M.I., 2013. Prevalence and risk factors for infection of bovine tuberculosis in indigenous cattle in the Serengeti ecosystem, Tanzania, BMC Veterinary Research, 9, doi:10.1186/1746-6148-9-267
Kulldorf, M., 1997. A spatial scan statistic, Communications in Statistics - Theory and Methods, 26, 1481 – 1496
Matope, G., Bhebhe, E., Muma, J.B., Lund, A. and Skjerve, E., 2010. Herd-level factors for Brucella seropositivity in cattle reared in smallholder dairy farms of Zimbabwe, Preventive Veterinary Medicine, 94, 213-221
Mazet, J.A., Clifford, D.L., Coppolillo, P.B., Deolalikar, A.B..., Erickson, J.D. and Kazwala, R.R., 2009. A "one health" approach to address emerging zoonoses: the HALI project in Tanzania, PLoS Medicine 6, e1000190. doi:10.1371/journal.pmed.1000190
McDermott, J.J. and Arimi, S.M., 2002. Brucellosis in sub-Saharan Africa: epidemiology, control and impact, Veterinary Microbiology, 90, 111-134
Mfinanga, S.G., Morkve, O., Kazwala, R.R., Cleaveland, S., Sharp, J.M., Shirima, G. and Nilsen, R., 2003. The role of livestock keeping in tuberculosis trends in Arusha, Tanzania, International Journal of Tuberculosis and Lung Disease, 7, 695-704
Motsi, T.R., Tichiwangana, S.C., Matope, G. and Mukarati, N.L., 2013. A serological survey of brucellosis in wild ungulate species from five game parks in Zimbabwe, Onderstepoort Journal of Veterinary Research, 80, doi:10.4102/ojvr.v80i1.586.
Muma, J., Samui, K., Siamudaala, V., Oloya, J., Matope, G., Omer, M., Munyeme, M., Mubita, C. and Skjerve, E., 2006. Prevalence of antibodies to Brucella spp. and individual risk factors of infection in traditional cattle, goats and sheep reared in livestock–wildlife interface areas of Zambia, Tropical Animal Health and Production, 38, 195-206
Munyeme, M., Muma, J., Samui, K., Skjerve, E., Nambota, A., Phiri, I., Rigouts, L. and Tryland, M., 2009. Prevalence of bovine tuberculosis and animal level risk factors for indigenous cattle under different grazing strategies in the livestock/wildlife interface areas of Zambia, Tropical Animal Health and Production, 41, 345-352
Munyeme, M. and Munang'andu, H.M., 2011. A review of bovine tuberculosis in the Kafue basin ecosystem, Veterinary Medicine International 2011, 918743. doi:10.4061/2011/918743
Mwakalila, S., 2011: Assessing the hydrological conditions of the Usangu Wetlands in Tanzania, Journal of Water Resource and Protection, 3, 876-882
Mwakapuja, R.S., Makondo, Z.E., Malakalinga, J., Bryssinckx, W., Mdegela, R.H., Moser, I., Kazwala, R.R. and Tanner, M., 2013. Prevalence and significant geospatial clusters of bovine tuberculosis infection at livestock-wildlife interface ecosystem in Eastern Tanzania, Tropical Animal Health and Production, 45, 1223-30
Oloya, J., Muma, J.B., Opuda-Asibo, J., Djønne, B., Kazwala, R. and Skjerve, E. 2007. Risk factors for herd-level bovine-tuberculosis seropositivity in transhumant cattle in Uganda, Preventive Veterinary Medicine, 80, 318-329
Swai, E.S. and Schoonman, L., 2010. The use of rose bengal plate test to asses cattle exposure to Brucella infection in traditional and smallholder dairy production systems of Tanga region of Tanzania, Veterinary Medicine International 2010. doi:10.4061/2010/837950
Swai, E.S. and Schoonman, L., 2012. Differences in prevalence of tuberculosis in indigenous and crossbred cattle under extensive and intensive management systems in Tanga region of Tanzania, Tropical Animal Health and Production, 44, 459-465
Acknowledgments
This research was part of the Health for Animals and Livelihood Improvement (HALI) Project, a One Health project addressing zoonotic diseases in Tanzania, and was funded by the Global Livestock Collaborative Research Support Program by the Office of Agriculture, Bureau for Economic Growth, Agriculture and Trade, United States Agency for International Development under the terms of Grant No. PCE-G-00-98-00036-00. Special thanks go to Howard Kombe who assisted with household interviews and testing of cattle; to Jonas Fitwangile, Ali Kitime, and Kuleng’wa Ndaki Lukiko for laboratory assistance; and to Laurel Beckett, Esthe Geraghty, and Kate Thomas for their advice on statistical analysis and mapping.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Relevant survey questions
-
1.
Have there been any animals that have suffered a chronic debilitating illness for last 12 months? (Y/N)
-
2.
Do you graze animals beyond the village boundary? (Y/N)
-
3.
Do you allow your herd to mix with those from other bomas? (Y/N)
Rights and permissions
About this article
Cite this article
Roug, A., Clifford, D., Mazet, J. et al. Spatial predictors of bovine tuberculosis infection and Brucella spp. exposure in pastoralist and agropastoralist livestock herds in the Ruaha ecosystem of Tanzania. Trop Anim Health Prod 46, 837–843 (2014). https://doi.org/10.1007/s11250-014-0574-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-014-0574-9