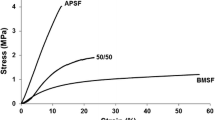
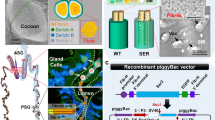
Abstract
The domesticated silkworm, Bombyx mori, is a fundamental insect for silk industry. Silk is obtained from cocoons, protective envelopes produced during pupation and composed of single raw silk filaments secreted by the insect silk glands. Currently, silk is used as a textile fibre and to produce new materials for technical and biomedical applications. To enhance the use of both fabrics and silk-based materials, great efforts to obtain silk with antimicrobial properties have been made. In particular, a convincing approach is represented by the enrichment of the textile fibre with antimicrobial peptides, the main effectors of the innate immunity. To this aim, silkworm-based transgenic techniques appear to be cost-effective strategies to obtain cocoons in which antimicrobial peptides are integrated among the silk proteins. Recently, cocoons transgenic for a recombinant silk protein conjugated to the silkworm Cecropin B antimicrobial peptide were obtained and showed enhanced antibacterial properties (Li et al. in Mol Biol Rep 42:19–25, https://doi.org/10.1007/s11033-014-3735-z, 2015a). In this work we used the piggyBac-mediated germline transformation to generate several transgenic B. mori lines able to overexpress Cecropin B or Moricin antimicrobial peptides at the level of the silk gland. The derived cocoons were characterised by increased antimicrobial properties and the resulting silk fibre was able to inhibit the bacterial growth of the Gram-negative Escherichia coli. Our results suggest that the generation of silkworm overexpressing unconjugated antimicrobial peptides in the silk gland might represent an additional strategy to obtain antimicrobial peptide-enriched silk, for the production of new silk-based materials.



Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
ASTM International (2013) ASTM E2149-13a, standard test method for determining the antimicrobial activity of antimicrobial agents under dynamic contact conditions. West Conshohocken. https://doi.org/10.1520/e2149. www.astm.org
Atrouni AA, Joly-Guillou ML, Hamze M, Kempf M (2016) Reservoirs of non-baumannii Acinetobacter species. Front Microbiol 7:49. https://doi.org/10.3389/fmicb.2016.00049
Baggio F, Bozzato A, Benna C, Leonardi E, Romoli O, Cognolato M, Tosatto SC, Costa R (2013) Sandrelli F (2013) 2mit, an intronic gene of Drosophila melanogaster timeless2, is involved in behavioral plasticity. PLoS ONE 8:e76351. https://doi.org/10.1371/journal.pone.0076351
Bai L, Zhu L, Min S, Liu L, Cai Y, Yao J (2008) Surface modification and properties of Bombyx mori silk fibroin films by antimicrobial peptide. Appl Surf Sci 254:2988–2995. https://doi.org/10.1016/j.apsusc.2007.10.049
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. https://doi.org/10.1038/nrmicro1098
Callaway JE, Lai J, Haselbeck B, Baltaian M, Bonnesen SP, Weickmann J, Wilcox G, Lei SP (1993) Modification of the C terminus of cecropin is essential for broad-spectrum antimicrobial activity. Antimicrob Agents Chemother 37:1614–1619. https://doi.org/10.1128/AAC.37.8.1614
Cao TT, Zhang YQ (2016) Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater Sci Eng C Mater Biol Appl 61:940–952. https://doi.org/10.1016/j.msec.2015.12.082
Cappellozza S (inventor) (2004) CREA, assignee. Patent IT 000155
Cappellozza S, Saviane A (inventors) (2015) CREA, assignee. Patent IT 1416868 (2015)
Cappellozza S, Saviane A, Tettamanti G, Squadrin M, Vendramin E, Paolucci P, Franzetti E, Squartini A (2011) Identification of Enterococcus mundtii as a pathogenic agent involved in the “flacherie” disease in Bombyx mori L. larvae reared on artificial diet. J Invertebr Pathol 106:386–393. https://doi.org/10.1016/j.jip.2010.12.007
Cheng T, Zhao P, Liu C, Xu P, Gao Z, Xia Q, Xiang Z (2006) Structures, regulatory regions, and inductive expression patterns of antimicrobial peptide genes in the silkworm, Bombyx mori. Genomics 87:356–365. https://doi.org/10.1016/j.ygeno.2005.11.018
Costa F, Carvalho IF, Montelaro RC, Gomes P, Martins MCL (2011) Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater 7:1431–1440. https://doi.org/10.1016/j.actbio.2010.11.005
Doughari HJ, Ndakidemi PA, Human IS, Benade S (2011) The ecology, biology and pathogenesis of Acinetobacter spp.: an overview. Microbes Environ 26:101–112. https://doi.org/10.1264/jsme2.ME10179
Duan J, Li R, Cheng D, Fan W, Zha X, Cheng T, Wu Y, Wang J, Mita K, Xiang Z, Xia Q (2010) SilkDB v2.0: a platform for silkworm (Bombyx mori) genome biology. Nucleic Acids Res 38:D453–D456. https://doi.org/10.1093/nar/gkp801
Fang SM, Hu BL, Zhou QZ, Yu QY, Zhang Z (2015) Comparative analysis of the silk gland transcriptomes between the domestic and wild silkworms. BMC Genomics 16:60. https://doi.org/10.1186/s12864-015-1287-9
Fraser MJ Jr (2012) Insect transgenesis: current applications and future prospects. Annu Rev Entomol 57:267–289. https://doi.org/10.1146/annurev.ento.54.110807.090545
Gao Y, Cranston R (2008) Recent advances in antimicrobial treatments of textiles. Text Res J 78:60–72. https://doi.org/10.1177/0040517507082332
Grzelak K (1995) Control of expression of silk protein genes. Comp Biochem Physiol B Biochem Mol Biol 110:671–681. https://doi.org/10.1016/0305-0491(94)00215-G
Gtari M, Ghodhbane-Gtari F, Nouioui I, Beauchemin N, Tisa LS (2012) Phylogenetic perspectives of nitrogen-fixing actinobacteria. Arch Microbiol 194:3–11. https://doi.org/10.1007/s00203-011-0733-6
Hao L, Wu J, Lin S, Tian T, Qi J, Yang J, Wang Z (2011) Immobilized antibacterial peptides on polyethylene terephthalate non-woven fabrics and antibacterial activity evaluation. Text Res J 81:1977–1982. https://doi.org/10.1177/0040517510402126
Hara S, Yamakawa M (1995) Moricin, a novel type of antibacterial peptide isolated from the silkworm, Bombyx mori. J Biol Chem 270:29923–29927. https://doi.org/10.1074/jbc.270.50.29923
Hu H, Wang C, Guo X, Li W, Wang Y, He Q (2013) Broad activity against porcine bacterial pathogens displayed by two insect antimicrobial peptides Moricin and Cecropin B. Mol Cells 35:106–114. https://doi.org/10.1007/s10059-013-2132-0
Inoue S, Tanaka K, Arisaka F, Kimura S, Ohtomo K, Mizuno S (2000) Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J Biol Chem 275:40517–40528. https://doi.org/10.1074/jbc.M006897200
Kamjam M, Sivalingam P, Deng Z, Hong K (2017) Deep sea Actimomycetes and their secondary metabolites. Front Microbiol 8:760. https://doi.org/10.3389/fmicb.2017.00760
Kaneko Y, Tanaka H, Ishibashi J, Iwasaki T, Yamakawa M (2008) Gene expression of a novel defensin antimicrobial peptide in the silkworm, Bombyx mori. Biosci Biotechnol Biochem 72:2353–2361. https://doi.org/10.1271/bbb.80263
Koh LD, Yuan Cheng Y, Tenga CP, Khina YW, Loha XJ, Teea SY, Lowa M, Yea E, Yua HD, Zhang YW, Hana MY (2015) Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci 46:86–110. https://doi.org/10.1016/j.progpolymsci.2015.02.001
Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS (2010) Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA 107:8111–8116. https://doi.org/10.1073/pnas.1003056107
Kundu B, Rajkhowa R, Kundu SC, Wang X (2013) Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev 65:457–470. https://doi.org/10.1016/j.addr.2012.09.043
Li Z, Jiang Y, Cao G, Li J, Xue R, Gong C (2015a) Construction of transgenic silkworm spinning antibacterial silk with fluorescence. Mol Biol Rep 42:19–25. https://doi.org/10.1007/s11033-014-3735-z
Li AB, Kluge JA, Guziewicz NA, Omenetto FG, Kaplan DL (2015b) Silk-based stabilization of biomacromolecules. J Control Release 219:416–430. https://doi.org/10.1016/j.jconrel.2015.09.03
Liu X, Lin T, Peng B, Wang X (2012) Antibacterial activity of capsaicin-coated wool fabric. Text Res J 82:584–590. https://doi.org/10.1177/0040517511426608
Melo AL, Soccol VT, Soccol CR (2016) Bacillus thuringiensis: mechanism of action, resistance, and new applications: a review. Crit Rev Biotechnol 36:317–326. https://doi.org/10.3109/07388551.2014.960793
Montali A, Romanelli D, Cappellozza S, Grimaldi A, de Eguileor M, Tettamanti G (2017) Timing of autophagy and apoptosis during posterior silk gland degeneration in Bombyx mori. Arthropod Struct Dev 46:518–528. https://doi.org/10.1016/j.asd.2017.05.003
Nadiger VG, Shukla RS (2016) Antibacterial properties of silk fabric treated with silver nanoparticles. J Text Inst 107:1543–1553. https://doi.org/10.1080/00405000.2015.1129756
Nicholson WL (2002) Roles of Bacillus endospores in the environment. Cell Mol Life Sci 59:410–416. https://doi.org/10.1007/s00018-002-8433-7
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. https://doi.org/10.1093/nar/29.9.e45
Ponnuvel KM, Subhasri N, Sirigineedi S, Murthy GN, Vijayaprakash NB (2010) Molecular evolution of the cecropin multigene family in silkworm Bombyx mori. Bioinformation 5:97–103
Pritchard EM, Kaplan DL (2011) Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv 8:797–811. https://doi.org/10.1517/17425247.2011.568936
Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL (2011) Materials fabrication from Bombyx mori silk fibroin. Nat Protoc 6:1612–1631. https://doi.org/10.1038/nprot.2011.379
Romoli O, Saviane A, Bozzato A, D’Antona P, Tettamanti G, Squartini A, Cappellozza S, Sandrelli F (2017) Differential sensitivity to infections and antimicrobial peptide-mediated immune response in four silkworm strains with different geographical origin. Sci Rep 7:1048. https://doi.org/10.1038/s41598-017-01162-z
Rosselli R, Romoli O, Vitulo N, Vezzi A, Campanaro S, de Pascale F, Schiavon R, Tiarca M, Poletto F, Concheri G, Valle G, Squartini A (2016) Direct 16S rRNA-seq from bacterial communities: a PCR-independent approach to simultaneously assess microbial diversity and functional activity potential of each taxon. Sci Rep 6:32165. https://doi.org/10.1038/srep32165
Sandrelli F, Cappellozza S, Benna C, Saviane A, Mastella A, Mazzotta GM, Moreau S, Pegoraro M, Piccin A, Zordan MA, Cappellozza L, Kyriacou CP, Costa R (2007) Phenotypic effects induced by knock-down of the period clock gene in Bombyx mori. Genet Res 89:73–84. https://doi.org/10.1017/S0016672307008713
Schaegger H (2006) Tricine-SDS-PAGE. Nat Protoc 1:16–22. https://doi.org/10.1038/nprot.2006.4
Schaunig C, Kopera D (2017) Silk textile with antimicrobial AEM5772/5 (Dermasilk): a pilot study with positive influence on acne vulgaris on the back. Int J Dermatol 56:589–591. https://doi.org/10.1111/ijd.13541
Sezutsu H, Uchino K, Kobayashi I, Tatematsu K, Iizuka T, Yonemura N, Tamura T (2009) Conservation of fibroin gene promoter function between the domesticated silkworm Bombyx mori and the wild silkmoth Antheraea yamamai. J Insect Biotechnol Sericol 78:1–10
Shimomura M, Minami H, Suetsugu Y, Ohyanagi H, Satoh C, Antonio B, Nagamura Y, Kadono-Okuda K, Kajiwara H, Sezutsu H, Nagaraju J, Goldsmith MR, Xia Q, Yamamoto K, Mita K (2009) KAIKObase: an integrated silkworm genome database and data mining tool. BMC Genomics 10:486. https://doi.org/10.1186/1471-2164-10-486
Song DW, Kim SH, Kim HH, Lee KH, Ki CS, Park YH (2016) Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: implications for wound healing. Acta Biomater 39:146–155. https://doi.org/10.1016/j.actbio.2016.05.008
Takasu Y, Hata T, Uchino K, Zhang Q (2010) Identification of Ser2 proteins as major sericin components in the non-cocoon silk of Bombyx mori. Insect Biochem Mol Biol 40:339344. https://doi.org/10.1016/j.ibmb.2010.02.010
Tamura T, Thibert C, Royer C, Kanda T, Eappen A, Kamba M, Kômoto N, Thomas JL, Mauchamp B, Chavancy G, Shirk P, Fraser M, Prudhomme JC, Couble P (2000) Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 18:81–84. https://doi.org/10.1038/71978
Tanaka H, Ishibashi J, Fujita K, Nakajima Y, Sagisaka A, Tomimoto K, Suzuki N, Yoshiyama M, Kaneko Y, Iwasaki T, Sunagawa T, Yamaji K, Asaoka A, Mita K, Yamakawa M (2008) A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem Mol Biol 38:1087–1110. https://doi.org/10.1016/j.ibmb.2008.09.001
Tiago I, Chung AP, Veríssimo A (2004) Bacterial diversity in a nonsaline alkaline environment: heterotrophic aerobic populations. Appl Environ Microbiol 70:7378–7387. https://doi.org/10.1128/AEM.70.12.7378-7387.2004
Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D (2007) Genomics of Actinobacteria: tracing the evolutionary history of an ancient Phylum. Microbiol Mol Biol Rev 71:495–548. https://doi.org/10.1128/MMBR.00005-07
Weng H, Pan A, Yang L, Zhang C, Liu Z, Zhang D (2004) Estimating number of transgene copies in transgenic rapeseed by real-time PCR assay withHMG I/Y as an endogenous reference gene. Plant Mol Biol Rep 22:289. https://doi.org/10.1007/BF02773139
Wilson D, Valluzzi R, Kaplan D (2000) Conformational transition in model silk peptides. Biophys J 78:2690–2701. https://doi.org/10.1016/S0006-3495(00)76813-5
Wöltje M, Böbel M (2016) Natural biodegradable medical polymers: silk. In: Zhang XC (ed) Science and principles of biodegradable and bioresorbable medical polymers, 1st edn. Elsevier, Netherlands, pp 351–376
Xu H, O’Brochta D (2015) Advanced technologies for genetically manipulating the silkworm Bombyx mori, a model Lepidopteran insect. Proc Biol Sci 282:20150487. https://doi.org/10.1098/rspb.2015.0487
Yamano Y, Matsumoto M, Inoue K, Kawabata T, Morishima I (1994) Cloning of cDNAs for cecropins A and B, and expression of the genes in the silkworm, Bombyx mori. Biosci Biotechnol Biochem 58:1476–1478. https://doi.org/10.1271/bbb.58.1476
Yang W, Cheng T, Ye M, Deng X, Yi H, Huang Y, Tan X, Han D, Wang B, Xiang Z, Cao Y, Xia Q (2011) Functional divergence among silkworm antimicrobial peptide paralogs by the activities of recombinant proteins and the induced expression profiles. PLoS ONE 6:e18109. https://doi.org/10.1371/journal.pone.0018109
Yi HY, Chowdhury M, Huang YD, Yu XQ (2014) Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol 98:5807–5822. https://doi.org/10.1007/s00253-014-5792-6
Yua D, Kanga G, Tiana W, Lina L, Wanga W (2015) Preparation of conductive silk fabric with antibacterial properties by electroless silver plating. Appl Surf Sci 357:1157–1162. https://doi.org/10.1016/j.apsusc.2015.09.074
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395. https://doi.org/10.1038/415389a
Zhou CZ, Confalonieri F, Medina N, Zivanovic Y, Esnault C, Yant T, Jacquet M, Janin J, Duguet M, Perasso R, Li ZG (2000) Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res 28:2413–2419. https://doi.org/10.1093/nar/28.12.2413
Zhou CZ, Confalonieri F, Jacquet M, Perasso R, Li ZG, Janin J (2001) Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins 44:119–122. https://doi.org/10.1002/prot.1078
Acknowledgements
This work was supported by grants from Regione Lombardia to G.T. and G.F. in the framework of the Project ID 30141940 “SILKBIOTECH: biotechnological production of antimicrobial silk” (MIUR-Regione Lombardia 2012–2015) and by grants from the companies and institutions (Ratti srl, Res Pharma srl, Bioengineering laboratories srl, Tintoria Clerici srl, University of Insubria, Innovhub-SSI, Silk Area) participating to the same project as S.C.; from CARIPARO (Progetti di Eccellenza 2011/12) to S.C, F.S. and G.T. and from the Università degli Studi di Padova (CPDA154301) to F.S. A.B. was supported by a postdoctoral fellowship from Università degli Studi di Padova (CPDR100470). We are grateful to NARO (Japan) and EMBL (Germany) for providing the pBacFibH-Gal4/3xP3DsRed and pETM-22 plasmids, respectively. The authors wish to thank Paola D’Antona who participated in early experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
All the authors declared that they have no conflict of interest.
Ethical statements
Our study complies with institutional standards on silkworm research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saviane, A., Romoli, O., Bozzato, A. et al. Intrinsic antimicrobial properties of silk spun by genetically modified silkworm strains. Transgenic Res 27, 87–101 (2018). https://doi.org/10.1007/s11248-018-0059-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-018-0059-0