Abstract
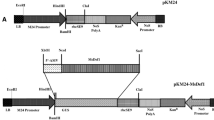
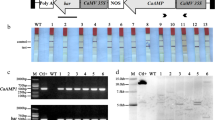
Two novel antifungal hevein-like peptides, SmAMP1.1a and SmAMP2.2a, were previously isolated from seeds of Stellaria media. It has been established that these peptides accumulate in this weed as a result of proteolysis of two propeptides, pro-SmAMP1 and pro-SmAMP2. The primary structure of these propeptides is unique; in addition to having a signal peptide and negatively charged C-terminus, each of these structures consists of two hevein-like peptides of different length separated by a space rather than a single peptide. In this work, we demonstrated that the expression of the pro-SmAMP1 and pro-SmAMP2 genes was tissue-specific and increased substantially under exposure to fungal infection. To elucidate whether S. media has any advantages in defending against phytopathogens due to its unusual structure of pro-SmAMP1 and pro-SmAMP2, on the basis of the pro-SmAMP1 gene, we created three genetic constructs. Arabidopsis and tobacco plants were subsequently transformed with these constructs. Transgenic plants bearing the full-length pro-SmAMP1 gene exhibited the best resistance to the phytopathogens Bipolaris sorokiniana and Thielaviopsis basicola. The resistance of S. media plants to phytopathogenic fungi was likely due to the fungal-inducible expression of pro-SmAMP1 and pro-SmAMP2 genes, and due to the specific features of the primary structure of the corresponding propeptides. As a result of the processing of these propeptides, two different antimicrobial peptides were released simultaneously. Based on our results, we conclude that the genes for antimicrobial peptides from S. media may be promising genetic tools for the improvement of plant resistance to fungal diseases.



Similar content being viewed by others
References
Aerts AM, Francois IE, Cammue BP, Thevissen K (2008) The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci 65:2069–2079
Beintema JJ (1994) Structural features of plant chitinases and chitin-binding proteins. FEBS Lett 350:159–163
Benko-Iseppon AM, Galdino SL, Calsa T Jr, Kido EA, Tossi A, Belarmino LC, Crovella S (2010) Overview on plant antimicrobial peptides. Curr Protein Pept Sci 11:181–188
Broekaert I, Lee HI, Kush A, Chua NH, Raikhel N (1990) Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis). Proc Natl Acad Sci USA 87:7633–7637
Broekaert WF, Marien W, Terras FR, De Bolle MF, Proost P, Van Damme J, Dillen L, Claeys M, Rees SB, Vanderleyden J et al (1992) Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry 31:4308–4314
Broekaert WF, Terras FR, Cammue BP, Osborn RW (1995) Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol 108:1353–1358
Broekaert W, Cammue B, De Bolle MF, Thevissen K, De Samblanx GW, Osborn R (1997) Antimicrobial peptides from plants. Crit Rev Plant Sci 16:297–323
Cammue BP, De Bolle MF, Terras FR, Proost P, Van Damme J, Rees SB, Vanderleyden J, Broekaert WF (1992) Isolation and characterization of a novel class of plant antimicrobial peptides form Mirabilis jalapa L. seeds. J Biol Chem 267:2228–2233
Cammue BP, Thevissen K, Hendriks M, Eggermont K, Goderis IJ, Proost P, Van Damme J, Osborn RW, Guerbette F, Kader JC et al (1995) A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteins. Plant Physiol 109:445–455
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Craik DJ, Daly NL, Bond T, Waine C (1999) Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol 294:1327–1336
De Bolle MF, Osborn RW, Goderis IJ, Noe L, Acland D, Hart CA, Torrekens S, Van Leuven F, Broekaert WF (1996) Antimicrobial peptides from Mirabilis jalapa and Amaranthus caudatus: expression, processing, localization and biological activity in transgenic tobacco. Plant Mol Biol 31:993–1008
De Lucca AJ, Cleveland TE, Wedge DE (2005) Plant-derived antifungal proteins and peptides. Can J Microbiol 51:1001–1014
Epple P, Apel K, Bohlmann H (1997) Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell 9:509–520
Escalona VH, Aguayo E, Martínez-Hernández GB, Artés F (2010) UV-C doses to reduce pathogen and spoilage bacterial growth in vitro and in baby spinach. Postharvest Biol Technol 56:223–231
Fernandez de Caleya R, Gonzalez-Pascual B, Garcia-Olmedo F, Carbonero P (1972) Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Microbiol 23:998–1000
Francois IEJA, De Bolle MFC, Dwyer G, Goderis IJWM, Woutors PFJ, Verhaert PD, Proost P, Schaaper WMM, Cammue BPA, Broekaert WF (2002) Transgenic expression in Arabidopsis of a polyprotein construct leading to production of two different antimicrobial proteins. Plant Physiol 128:1346–1358
Gao AG, Hakimi SM, Mittanck CA, Wu Y, Woerner BM, Stark DM, Shah DM, Liang J, Rommens CM (2000) Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat Biotechnol 18:1307–1310
Garcia-Olmedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P (1998) Plant defense peptides. Biopolymers 47:479–491
Guo G, Wang HX, Ng TB (2009) Pomegranin, an antifungal peptide from pomegranate peels. Protein Pept Lett 16:82–85
Hammami R, Ben Hamida J, Vergoten G, Fliss I (2009) PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acids Res 37(Database issue):D963–968
Horsch RB, Rogers SG, Fraley RT (1985) Transgenic plants. Cold Spring Harb Symp Quant Biol 50:433–437
Huang X, Xie W, Gong Z (2000) Characteristics and antifungal activity of a chitin binding protein from Ginkgo biloba. FEBS Lett 478:123–126
Huang RH, Xiang Y, Liu XZ, Zhang Y, Hu Z, Wang DC (2002) Two novel antifungal peptides distinct with a five-disulfide motif from the bark of Eucommia ulmoides Oliv. FEBS Lett 521:87–90
Isken O, Maquat LE (2007) Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 21:1833–1856
Jha S, Chattoo BB (2010) Expression of a plant defensin in rice confers resistance to fungal phytopathogens. Transgenic Res 19:373–384
Jha S, Tank HG, Prasad BD, Chattoo BB (2009) Expression of Dm-AMP1 in rice confers resistance to Magnaporthe oryzae and Rhizoctonia solani. Transgenic Res 18:59–69
Kang TJ, Yang MS (2004) Rapid and reliable extraction of genomic DNA from various wild-type and transgenic plants. BMC Biotechnol 4:20
Kiba A, Saitoh H, Nishihara M, Omiya K, Yamamura S (2003) C-terminal domain of a hevein-like protein from Wasabia japonica has potent antimicrobial activity. Plant Cell Physiol 44:296–303
Komakhin R, Komakhina V, Milyukova N, Goldenkova-Pavlova I, Fadina O, Zhuchenko A (2010) Transgenic tomato plants expressing recA and NLS-recA-licBM3 genes as a model for studying meiotic recombination. Russ J Genet 46:1440–1448
Koo JC, Lee SY, Chun HJ, Cheong YH, Choi JS, Kawabata S, Miyagi M, Tsunasawa S, Ha KS, Bae DW, Han CD, Lee BL, Cho MJ (1998) Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent antifungal activity. Biochim Biophys Acta 1382:80–90
Koo JC, Chun HJ, Park HC, Kim MC, Koo YD, Koo SC, Ok HM, Park SJ, Lee SH, Yun DJ, Lim CO, Bahk JD, Lee SY, Cho MJ (2002) Over-expression of a seed specific hevein-like antimicrobial peptide from Pharbitis nil enhances resistance to a fungal pathogen in transgenic tobacco plants. Plant Mol Biol 50:441–452
Lay FT, Anderson MA (2005) Defensins–components of the innate immune system in plants. Curr Protein Pept Sci 6:85–101
Lee OS, Lee B, Park N, Koo JC, Kim YH, Prasad DT, Karigar C, Chun HJ, Jeong BR, Kim DH, Nam J, Yun JG, Kwak SS, Cho MJ, Yun DJ (2003) Pn-AMPs, the hevein-like proteins from Pharbitis nil confers disease resistance against phytopathogenic fungi in tomato, Lycopersicum esculentum. Phytochemistry 62:1073–1079
Li SS, Claeson P (2003) Cys/Gly-rich proteins with a putative single chitin-binding domain from oat (Avena sativa) seeds. Phytochemistry 63:249–255
Lin P, Ng TB (2009) Brassiparin, an antifungal peptide from Brassica parachinensis seeds. J Appl Microbiol 106:554–563
Lipkin A, Anisimova V, Nikonorova A, Babakov A, Krause E, Bienert M, Grishin E, Egorov T (2005) An antimicrobial peptide Ar-AMP from amaranth (Amaranthus retroflexus L.) seeds. Phytochemistry 66:2426–2431
Marcus JP, Goulter KC, Green JL, Harrison SJ, Manners JM (1997) Purification, characterisation and cDNA cloning of an antimicrobial peptide from Macadamia integrifolia. Eur J Biochem 244:743–749
McManus AM, Nielsen KJ, Marcus JP, Harrison SJ, Green JL, Manners JM, Craik DJ (1999) MiAMP1, a novel protein from Macadamia integrifolia adopts a Greek key beta-barrel fold unique amongst plant antimicrobial proteins. J Mol Biol 293:629–638
Molina A, Segura A, Garcia-Olmedo F (1993) Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett 316:119–122
Muller PY, Janovjak H, Miserez AR, Dobbie Z (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32:1372–1379
Murashige T (1973) Nutrition of plant cells and organs in vitro. In Vitro 9:81–85
Nielsen KK, Nielsen JE, Madrid SM, Mikkelsen JD (1997) Characterization of a new antifungal chitin-binding peptide from sugar beet leaves. Plant Physiol 113:83–91
Odintsova TI, Vassilevski AA, Slavokhotova AA, Musolyamov AK, Finkina EI, Khadeeva NV, Rogozhin EA, Korostyleva TV, Pukhalsky VA, Grishin EV, Egorov TA (2009) A novel antifungal hevein-type peptide from Triticum kiharae seeds with a unique 10-cysteine motif. FEBS J 276:4266–4275
Parijs J, Broekaert W, Goldstein I, Peumans W (1991) Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 183:258–264
Patel SU, Osborn R, Rees S, Thornton JM (1998) Structural studies of Impatiens balsamina antimicrobial protein (Ib-AMP1). Biochemistry 37:983–990
Pieterse CM, van Loon LC (1999) Salicylic acid-independent plant defence pathways. Trends Plant Sci 4:52–58
Portieles R, Ayra C, Gonzalez E, Gallo A, Rodriguez R, Chacon O, Lopez Y, Rodriguez M, Castillo J, Pujol M, Enriquez G, Borroto C, Trujillo L, Thomma BP, Borras-Hidalgo O (2010) NmDef02, a novel antimicrobial gene isolated from Nicotiana megalosiphon confers high-level pathogen resistance under greenhouse and field conditions. Plant Biotechnol J 8:678–690
Raikhel NV, Lee HI, Broekaert WF (1993) Structure and function of chitin-binding proteins. Ann Rev Plant Physiol Plant Mol Biol 44:591–615
Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1:404–411
Shao F, Hu Z, Xiong YM, Huang QZ, Wang CG, Zhu RH, Wang DC (1999) A new antifungal peptide from the seeds of Phytolacca americana: characterization, amino acid sequence and cDNA cloning. Biochim Biophys Acta 1430:262–268
Swathi Anuradha T, Divya K, Jami SK, Kirti PB (2008) Transgenic tobacco and peanut plants expressing a mustard defensin show resistance to fungal pathogens. Plant Cell Rep 27:1777–1786
Tailor RH, Acland DP, Attenborough S, Cammue BP, Evans IJ, Osborn RW, Ray JA, Rees SB, Broekaert WF (1997) A novel family of small cysteine-rich antimicrobial peptides from seed of Impatiens balsamina is derived from a single precursor protein. J Biol Chem 272:24480–24487
Terras FR, Schoofs HM, De Bolle MF, Van Leuven F, Rees SB, Vanderleyden J, Cammue BP, Broekaert WF (1992) Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem 267:15301–15309
Terras FR, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, Van Leuven F, Vanderleyden J et al (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Thevissen K, Osborn RW, Acland DP, Broekaert WF (2000) Specific binding sites for an antifungal plant defensin from Dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Mol Plant Microbe Interact 13:54–61
Van Damme EJ, Charels D, Roy S, Tierens K, Barre A, Martins JC, Rouge P, Van Leuven F, Does M, Peumans WJ (1999) A gene encoding a hevein-like protein from elderberry fruits is homologous to PR-4 and class V chitinase genes. Plant Physiol 119:1547–1556
Van den Bergh KP, Proost P, Van Damme J, Coosemans J, Van Damme EJ, Peumans WJ (2002) Five disulfide bridges stabilize a hevein-type antimicrobial peptide from the bark of spindle tree (Euonymus europaeus L.). FEBS Lett 530:181–185
Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1:641–646
Acknowledgments
This work was supported by a grant from the Russian Foundation for Basic Research (no. 08-04-135-73 ofi -c).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
R. Shukurov, R., D. Voblikova, V., Nikonorova, A.K. et al. Transformation of tobacco and Arabidopsis plants with Stellaria media genes encoding novel hevein-like peptides increases their resistance to fungal pathogens. Transgenic Res 21, 313–325 (2012). https://doi.org/10.1007/s11248-011-9534-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-011-9534-6