Abstract
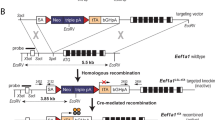
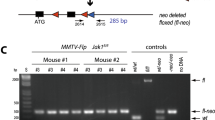
Although constitutive murine transgenic models have provided important insights into β-catenin signaling in tissue morphogenesis and tumorigenesis, these models are unable to express activated β-catenin in a temporally controlled manner. Therefore, to enable the induction (and subsequent de-induction) of β-catenin signaling during a predetermined time-period or developmental stage, we have generated and characterized a TETO-ΔN89β-catenin responder transgenic mouse. Crossed with the MTB transgenic effector mouse, which targets the expression of the reverse tetracycline transactivator (rtTA) to the mammary epithelium, we demonstrate that the stabilized (and activated) form of β-catenin (ΔN89β-catenin) is expressed only in the presence doxycycline-activated rtTA in the mammary epithelial compartment. Furthermore, we show that transgene-derived ΔN89β-catenin elicits significant mammary epithelial proliferation and precocious alveologenesis in the virgin doxycycline-treated MTB/TETO-ΔN89β-catenin bitransgenic. Remarkably, deinduction of TETO-ΔN89β-catenin transgene expression (through doxycycline withdrawal) results in the reversal of these morphological changes. Importantly, continued activation of the TETO-ΔN89β-catenin transgene results in palpable mammary tumors (within 7–9 months) in the doxycycline-treated virgin MTB/TETO-ΔN89β-catenin bigenic but not in the same bitransgenic without doxycycline administration. Collectively, these mammary epithelial responses to ΔN89β-catenin expression agree with previous reports using conventional transgenesis and therefore confirm that ΔN89β-catenin functions as expected in this doxycycline-responsive bigenic system. In sum, our mammary gland studies demonstrate “proof-of-principle” for using the TETO-ΔN89β-catenin transgenic responder to activate (and then de-activate) β-catenin signaling in any tissue of interest in a spatiotemporal specific fashion.







Similar content being viewed by others
References
Aberle H, Bauer A, Stappert J et al (1997) Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16:3797–3804
Behrens J, von Kries JP, Kuhl M et al (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638–642
Behrens J, Jerchow BA, Wurtele M et al (1998) Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280:596–599
Bernstein L, Ross RK (1993) Endogenous hormones and breast cancer risk. Epidemiol Rev 15:48–65
Bierie B, Nozawa M, Renou JP et al (2003) Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene 22:3875–3887
Blakely CM, Sintasath L, D’Cruz CM et al (2005) Developmental stage determines the effects of MYC in the mammary epithelium. Development 132:1147–1160
Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305
Chang H, Guillou F, Taketo MM et al (2009) Overactive beta-catenin signaling causes testicular sertoli cell tumor development in the mouse. Biol Reprod 81:842–849
D’Cruz CM, Gunther EJ, Boxer RB et al (2001) c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med 7:235–239
Fernandez-Valdivia R, Mukherjee A, Creighton CJ et al (2008) Transcriptional Response of the Murine Mammary Gland to Acute Progesterone Exposure. Endocrinology 149:6236–6250
Fernandez-Valdivia R, Mukherjee A, Ying Y et al (2009) The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol 328:127–139
Fodde R, Brabletz T (2007) Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19:150–158
Gounari F, Signoretti S, Bronson R et al (2002) Stabilization of beta-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelia. Oncogene 21:4099–4107
Grigoryan T, Wend P, Klaus A et al (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22:2308–2341
Gumbiner BM (1995) Signal transduction of beta-catenin. Curr Opin Cell Biol 7:634–640
Gunther EJ, Belka GK, Wertheim GB et al (2002) A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. Faseb J 16:283–292
Guo Z, Dose M, Kovalovsky D et al (2007) Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood 109:5463–5472
Harada N, Tamai Y, Ishikawa T et al (1999) Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 18:5931–5942
Hatsell S, Rowlands T, Hiremath M et al (2003) Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8:145–158
He TC, Sparks AB, Rago C et al (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Hiremath M, Lydon JP, Cowin P (2007) The pattern of beta-catenin responsiveness within the mammary gland is regulated by progesterone receptor. Development 134:3703–3712
Imbert A, Eelkema R, Jordan S et al (2001) Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol 153:555–568
Jeong JW, Lee HS, Franco HL et al (2009) Beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 28:31–40
Kaplan E, Meier P (1958) Non-parametric estimation for incomplete observation. J Am Stat Assoc 53:457–471
Leder A, Pattengale PK, Kuo A et al (1986) Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell 45:485–495
Lewandoski M (2001) Conditional control of gene expression in the mouse. Nat Rev Genet 2:743–755
Liu C, Li Y, Semenov M et al (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837–847
Lydon JP, Ge G, Kittrell FS et al (1999) Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res 59:4276–4284
MacMahon B, Cole P, Lin TM et al (1970) Age at first birth and breast cancer risk. Bull World Health Organ 43:209–221
Marchant J (1955) Influence of pregnancy and lactation on the incidence of mammary carcinoma induced with methylcholanthrene in female mice of the “IF” strain. J Pathol Bacteriol 70:415–418
Medina D, Smith GH (1999) Chemical carcinogen-induced tumorigenesis in parous, involuted mouse mammary glands. J Natl Cancer Inst 91:967–969
Miyoshi K, Hennighausen L (2003) Beta-catenin: a transforming actor on many stages. Breast Cancer Res 5:63–68
Mukherjee A, Soyal SM, Fernandez-Valdivia R et al (2007) Targeting reverse tetracycline-dependent transactivator to murine mammary epithelial cells that express the progesterone receptor. Genesis 45:639–646
Mukherjee A, Soyal SM, Li J et al (2010) Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEBJ 24:4408–4419
Munemitsu S, Albert I, Souza B et al (1995) Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A 92:3046–3050
Munemitsu S, Albert I, Rubinfeld B et al (1996) Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol 16:4088–4094
Peto R, Peto J (1972) Asymptotically efficient rank invariant test procedures. J R Stat Soc A 135:185–207
Peto R, Pike MC, Day NE et al (1980) Guidelines for simple, sensitive significance tests for carcinogenic effects in long-term animal experiments. IARC Monogr Eval Carcinog Risk Chem Hum Suppl 2:311–426
Polakis P (1999) The oncogenic activation of beta-catenin. Curr Opin Genet Dev 9:15–21
Reynolds SD, Zemke AC, Giangreco A et al (2008) Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells 26:1337–1346
Russo J, Tay LK, Russo IH (1982) Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat 2:5–73
Sawyers CL (2009) Shifting paradigms: the seeds of oncogene addiction. Nat Med 15:1158–1161
Shimizu H, Julius MA, Giarre M et al (1997) Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ 8:1349–1358
Shtutman M, Zhurinsky J, Simcha I et al (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 96:5522–5527
Teissedre B, Pinderhughes A, Incassati A et al (2009) MMTV-Wnt1 and -DeltaN89beta-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS One 4:e4537
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426
Teuliere J, Faraldo MM, Deugnier MA et al (2005) Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development 132:267–277
Wang TC, Cardiff RD, Zukerberg L et al (1994) Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369:669–671
Wang Y, Krivtsov AV, Sinha AU et al (2010) The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 327:1650–1653
Yost C, Torres M, Miller JR et al (1996) The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10:1443–1454
Zechner D, Fujita Y, Hulsken J et al (2003) Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol 258:406–418
Acknowledgments
The authors thank Jie Han for her technical assistance and the Darwin Transgenic Core Facility, Baylor College of Medicine for its mouse embryo microinjection services. We are also grateful to Dr. Lewis A. Chodosh (Department of Cancer Biology, Abramson Family Cancer Research Institute, University of Pennsylvania School of Medicine, Philadelphia, PA) for furnishing the p-Tet-Splice cloning vector and the MTB effector mouse. This research was funded by Department of Defense grant: DOD-BC074763 and National Institutes of Health grant: R21-CA129905 to Pamela Cowin and by National Institutes of Health grant: CA077530 to John P. Lydon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, A., Soyal, S.M., Li, J. et al. A mouse transgenic approach to induce β-catenin signaling in a temporally controlled manner. Transgenic Res 20, 827–840 (2011). https://doi.org/10.1007/s11248-010-9466-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9466-6