Abstract
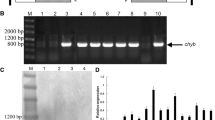
Nicotiana glauca is a tobacco species that forms flowers with carotenoid-pigmented petals, sepals, pistil, ovary and nectary tissue. The carotenoids produced are lutein, ß-carotene as well as some violaxanthin and antheraxanthin. This tobacco species was genetically modified for ketocarotenoid biosynthesis by transformation with a cyanobacterial crtO ketolase gene under the 35S CaMV promoter. In the transformants, ketocarotenoids were detected in both leaves and flowers. Although astaxanthin was not detected other ketocarotenoids such as 4′-ketolutein, echinenone, 3′-hydroxyechinenone and 4-ketozeaxanthin were present. Accumulation of ketocarotenoids in leaves decreased their photosynthetic efficiency moderately. Under the green house conditions used no impairment of growth and development compared to the wild type was observed. In the crtO-transformants, an unexpected up-regulation of total carotenoid biosynthesis in leaves and especially in flower petals was observed. This led to a total ketocarotenoid concentration in leaves of 136.6 (young) or 156.1 (older) μg/g dry weight and in petals of 165 μg/g dry weight. In our engineered plants, the ketocarotenoid pathway is one step short of astaxanthin. Strategies are discussed to improve N. glauca flowers as a biological system for astaxanthin.



Similar content being viewed by others
References
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721
Breitenbach J, Misawa N, Kajiwara S, Sandmann G (1996) Expression in Escherichia coli and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol Lett 140:241–246
Choi S, Matsuda S, Hoshino T, Peng X, Misawa N (2006) Characterization of bacterial β-carotene 3,3′-hydroxylases, CrtZ, and P450 in astaxanthin biosynthetic pathway and adinorubin production by gene combination in Escherichia coli. Appl Microbiol Biotechnol 72:1238–1246
Daniell H, Streatfield SJ, Wycoff K (2001) Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci 6:219–226
Davies BH (1976) Carotenoids. In: Goodwin TW (ed) Chemistry of plant pigments. Academic Press, London
Demmig-Adams B (1990) Carotenoids and photoprotection: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020:1–24
Eugster CH (1995) Chemical derivatization: microscale tests for the presence of common functional groups in carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids: isolation and analysis, vol 1A. Birkhäuser Verlag, Basel
Fernandez-Gonzalez B, Sandmann G, Vioque A (1997) A new type of asymmetrically acting beta-carotene ketolase is required for the synthesis of echinenone in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 272:9728–9733
Fraser PD, Shimada H, Misawa N (1998) Enzymic confirmation of reactions involved in routes to astaxanthin formation elucidated using a direct substrate in vitro assay. Eur J Biochem 252:229–236
Gerjets T, Sandmann G (2006) Ketocarotenoid formation in transgenic potato. J Exp Bot 57:3639–3645
Gerjets T, Sandmann M, Zhu C, Sandmann G (2007) Metabolic engineering of ketocarotenoid biosynthesis in leaves and flowers of tobacco species. Biotechnol J (Epub ahead of print)
Giddings G, Allison G, Brooks D, Carter A (2000) Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol 18:1151–1155
Giovannucci E (2002) Lycopene and prostate cancer risk. Methodological considerations in the epidemiologic literature. Pure Appl Chem 74:1427–1434
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Higuera-Ciapara I, Félix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 46:185–196
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SD, Fraley RT (1985) A simple and general method for transferring genes to plants. Science 227:1229–1231
Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H (2006) Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod 69:443–449
Landrum JT, Bone AR (2001) Lutein, zeaxanthin and the macular pigment. Arch Biochem Biophys 385:28–40
Lotan T, Hirschberg J (1995) Cloning and expression in Escherichia coli of the gene encoding beta-C-4-oxygenase, that converts beta-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett 364:125–128
Mackinney G (1941) Absorption of light by chlorophyll solutions, J Biol Chem 140:315–322
Mann V, Harker M, Pecker I, Hirschberg J (2000) Metabolic engineering of astaxanthin production in tobacco flowers. Nat Biotechnol 18:888–892
Misawa N, Satomi Y, Kondo K, Yokoyama A et al (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic. J Bacteriol 177:6575–6584
Morris WL, Ducreux LJM, Fraser PD, Millam S, Taylor MA (2006) Engineering ketocarotenoid biosynthesis in potato tubers. Metab Eng 8:253–263
Ojima K, Breitenbach J, Visser H, Setoguchi Y et al (2006) Cloning of the astaxanthin synthase gene from Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and its assignment as a beta-carotene 3-hydroxylase/4-ketolase. Mol Gen Genet 275:148–158
Ralley L, Enfissi EM, Misawa N, Schuch W et al (2004) Metabolic engineering of ketocarotenoid formation in higher plants. Plant J 39:477–486
Römer S, Lübeck J, Kauder F, Steiger S, Adomat C, Sandmann G (2002) Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab Eng 4:263–272
Rosen KM, Villa-Komaroff L (1990) An alternative method for the visualization of RNA in formaldehyde agarose gels. Focus 12:23–24
Sambrook J, Fritsch E, Maniatis F (eds) (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbour Laboratory Press, NY
Sandmann G (2002) Combinatorial biosynthesis of carotenoids in a heterologous host: a powerful approach for the biosynthesis of novel structures. Chem Biochem 3:629–635
Sandmann G, Römer S, Fraser PD (2006) Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metab Eng 8:291–302
Seybold A, Goodwin TW (1959) Occurence of astaxanthin in the flower petals of Adonis annua L Nature 184:1714–1715
Sharoni Y, Agbaria R, Amir H, Ben-Dor A et al (2003) Modulation of transcriptional activity by antioxidant carotenoids. Mol Asp Med 24:371–384
Stalberg K, Lindgren O, Ek B, Hoglund A (2003) Synthesis of ketocarotenoids in the seed of Arabidopsis thaliana. Plant J 36:771–779
Steiger S, Schäfer L, Sandmann G (1999) High-light upregulation of carotenoids and their antioxidative properties in the cyanobacterium Synechocystis PCC 6803. J Photochem Photobiol B 521:14–18
Suzuki S, Nishihara M, Nakatsuka T, Misawa N, Ogiwara I, Yamamura S (2007) Flower color alteration in Lotus japonicus by modification of the carotenoid biosynthetic pathway. Plant Cell Rep Electronically published ahead of print
Verwoerd TC, Dekker BM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17:2362
Acknowledgment
This work was partly supported by a grant from the National Natural Science Foundation of China (Grant no. 30370123) to C.Z.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, C., Gerjets, T. & Sandmann, G. Nicotiana glauca engineered for the production of ketocarotenoids in flowers and leaves by expressing the cyanobacterial crtO ketolase gene. Transgenic Res 16, 813–821 (2007). https://doi.org/10.1007/s11248-007-9151-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-007-9151-6