Abstract
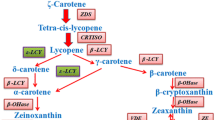
The accumulation of carotenoids in higher plants is regulated by the environment, tissue type and developmental stage. In Brassica napus leaves, β-carotene and lutein were the main carotenoids present while petals primarily accumulated lutein and violaxanthin. Carotenoid accumulation in seeds was developmentally regulated with the highest levels detected at 35–40 days post anthesis. The carotenoid biosynthesis pathway branches after the formation of lycopene. One branch forms carotenoids with two β rings such as β-carotene, zeaxanthin and violaxanthin, while the other introduces both β- and ε-rings in lycopene to form α-carotene and lutein. By reducing the expression of lycopene ε-cyclase (ε-CYC) using RNAi, we investigated altering carotenoid accumulation in seeds of B. napus. Transgenic seeds expressing this construct had increased levels of β-carotene, zeaxanthin, violaxanthin and, unexpectedly, lutein. The higher total carotenoid content resulting from reduction of ε-CYC expression in seeds suggests that this gene is a rate-limiting step in the carotenoid biosynthesis pathway. ε-CYC activity and carotenoid production may also be related to fatty acid biosynthesis in seeds as transgenic seeds showed an overall decrease in total fatty acid content and minor changes in the proportions of various fatty acids.





Similar content being viewed by others
References
Bartley GE, Scolnik PA (1995) Plant carotenoid: pigments for photoprotection, visual attraction, and human health. Plant Cell 7:1027–1038
Bassi R, Pineau B, Dainese P, Marquardt J (1993) Carotenoid-binding proteins of photosystem II. Eur J Biochem 212:297–303
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Botella-Pavía P, Rodríguez-Concepción M (2006) Carotenoid biotechnology in plants for nutritionally improved foods. Physiol Plant 126:369–381
Carpenter CD, Simon AE (1998) Preparation of RNA. In: Martinez-Zapater JM, Salinas J (eds) Methods in molecular biology, vol. 82. Arabidopsis protocols. Humana Press, Totowa, NJ, pp 85–89
Church G, Gilbert W (1984) Genome sequencing. Proc Natl Acad Sci USA 81:1991–1995
Cunningham FX Jr, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Cunningham FX Jr, Gantt E (2001) One ring or two? Determination of ring number in carotenoids by lycopene ε-cyclases. Proc Natl Acad Sci USA 98:2905–2910
Cuttriss AJ, Pogson BJ (2004) Carotenoids. In: Davies KM (ed) Plant pigments and their manipulation. CRC Press, Boca Raton, FL, pp 57–91
Demmig-Adams B, Gilmore AM, Adams WW III (1996) In vivo functions of carotenoids in higher plants. FASEB J 10:403–412
Demmig-Adams B, Adams WW III (2002) Antioxidants in photosynthesis and human nutrition. Science 298:2149–2153
Diretto G, Tavazza R, Welsch R, Pizzichini D, Mourgues F, Papacchioli V, Beyer P, Giuliano G (2006) Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biol 6:13
Diretto G, Welsch R, Tavazza R, Mourgues F, Pizzichini D, Beyer P, Giuliano G (2007) Silencing of beta-carotene hydroxylase increases total carotenoid and beta-carotene levels in potato tubers. BMC Plant Biol 7:11
Ducreux LJM, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, Taylor MA (2005) Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J Exp Bot 56:81–89
Fowler DB, Downey RK (1970) Lipid and morphological changes in developing rapeseed, Brassica napus. Can J Plant Sci 50:233–247
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Galau GA, Hughes DW, Dure L (1986) Abscisic-acid induction of cloned cotton late embryogenesis-abundant (lea) messenger-RNAs. Plant Mol Biol 7:155–170
Giuliano G, Bartley GE, Scolnik PA (1993) Regulation of carotenoid biosynthesis during tomato development. Plant Cell 5:379–387
Giuliano G, Aquilani R, Dharmapuri S (2000) Metabolic engineering of plant carotenoids. Trends Plant Sci 5:406–409
Goodwin TW (1980) The biochemistry of the carotenoids, 2nd edn., vol 1. Chapman & Hall, London, pp 377
Green BR, Durnford DG (1996) The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 47:685–714
Howitt CA, Pogson BJ (2006) Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ 29:435–445
Johnson-Flanagan AM, Huiwen Z, Geng X-M, Brown DCW, Nykiforuk CL, Singh S (1992) Frost, abscisic acid, and desiccation hasten embryo development in Brassica napus. Plant Physiol 99:700–706
Kirk JT, Tiliney-Bassett RA (1978) Proplastids, etioplasts, amyloplasts, chromoplasts and other plastids. In: Kirck ST, Tiliney-Bassett RA (eds) The plastids:their chemistry, structure, growth and inheritance. Elsevier/North Holland, Biomedical Press, Amsterdam, pp 217–239
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Aspects Med 26:459–516
Kuhlbrandt W, Wang DN, Fujiyoshi Y (1994) Atomic model of plant light-harvesting complex by electron crystallography. Nature 367:614–621
Landrum JT, Bone RA (2004) Dietary lutein and zeaxanthin: reducing the risk of macular degeneration. Agro Food Industry Hi-Tech 15:22–25
Lakshman MR, Okoh C (1993) Enzymatic conversion of all trans-beta-carotene to retinal. Meth Enzymol 214:256–269
Li L, Lu S, Cosman KM, Earle ED, Garvin DF, O’Neill J (2006) β-Carotene accumulation induced by cauliflower Or gene is not due to an increase capacity of biosynthesis. Phytochemistry 67:1177–1184
Li L, Van Eck J (2007) Metabolic engineering of carotenoid accumulation by creating a metabolic sink. Transgenic Res DOI 10.1007/s11248-007-9111-1
Lindgren L, Stahlberg KG, Hoglund AS (2003) Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in delayed germination and increased levels of carotenoids, chlorophyll, and abscisic acid. Plant Physiol 132:779–785
Mayne ST (1996) Beta-carotene, carotenoids and disease prevention in humans. FASEB J 10:690–701
Moloney MM, Walker JM, Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8:238–242
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48:109–136
Peter GF, Thornber JP (1991) Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-protein. J Biol Chem 266:16745–16754
Pogson BJ, McDonald K, Truong M, Britton G, DellaPenna D (1996) Arabidopsis carotenoid mutants demonstrate lutein is not essential for photosynthesis in higher plants. Plant Cell 8:1627–1639
Ravanello MP, Ke D, Alvarez J, Huang B, Shewmaker CK (2003) Coordinate expression of multiple bacterial carotenoid genes in canola leading to altered carotenoid production. Metab Eng 5:255–263
Rock CD, Zeevaart JAD (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci USA 88:7496–7499
Römer S, Lübeck J, Kauder F, Steiger S, Adomat C, Sandmann G (2002) Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab Eng 4:263–272
Rosati C, Aquilani R, Dharmapuri S, Pallara P, Marusic C, Tavazza R, Bouvier F, Camara B, Giuliano G (2000) Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J 24:413–419
Shewmaker CK, Sheey JA, Daley M, Colburn S, Ke DY (1999) Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J 20:401–412
Si P, Walton GH (2004) Determinants of oil concentration and seed yields in canola and Indian Mustard in the lower rainfall areas of Western Australia. Aust J Agric Res 55:367–377
Soeda Y, Konings MCJM, Vorst O, van Houwelingen AMML, Stoopen GM, Maliepaard CA, Kodde J, Bino RJ, Groot SPC, van der Geest AHM (2005) Gene expression programs during Brassica oleracea seed maturation, osmopriming, and germination are Indicators of progression of the germination process and the stress tolerance level. Plant Physiol 137:354–368
Stickforth P, Steiger S, Hess WR, Sandmann G (2003) A novel type of lycopene ε-cyclase in the marine cyanobacterium Prochlorococcus marinus MED4. Arch Microbiol 179:409–415
Taylor M, Ramsay G (2005) Carotenoid biosynthesis in plant storage organs: recent advances and prospects for improving plant food quality. Physiol Plant 124:143–151
Vishnevetsky M, Ovadis M, Vainstein A (1999) Carotenoid sequestration in plants: the role of carotenoid-associated proteins. Trends Plant Sci 4:232–235
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid –free) rice endosperm. Science 287:303–305
Young AJ (1993) Factors that affect the carotenoid composition of higher plants and algae. In: Young AJ, Britton G (eds) Carotenoids in photosynthesis. Chapman and Hall, London, pp 161–205
Young LW, Jalink H, Denkert R, Reaney MTJ (2006) Factors affecting the density of Brassica napus seeds. Seed Sci & Technol 34:633–645
Acknowledgements
We are grateful to Mr. Delwin Epp for technical assistance with B. napus tissue culture and Dr. Branimir Gjetvaj for assistance with the microarray analysis. We thank Drs. Kevin Falk, Kevin Rozwadowski and Bhinu V.S. for critical reading of the manuscript, and for helpful suggestions. Funding for this project was provided by the Saskatchewan Agriculture Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, B., Lydiate, D.J., Young, L.W. et al. Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Res 17, 573–585 (2008). https://doi.org/10.1007/s11248-007-9131-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-007-9131-x