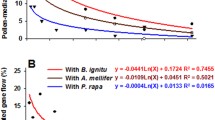
Abstract
The cultivation of genetically modified (GM) herbicide resistant oilseed rape (Brassica napus) has increased over the past few years. The transfer of herbicide resistance genes via pollen (gene flow) from GM crops to non-GM crops is of relevance for the realisation of co-existence of different agricultural cultivation forms as well as for weed management. Therefore the likelihood of pollen-mediated gene flow has been investigated in numerous studies. Despite the difficulty to compare different experiments with varying levels of outcrossing, we performed a literature search for world-wide studies on cross-fertilisation in fully fertile oilseed rape. The occurrence and frequency of pollen-mediated intraspecific gene flow (outcrossing rate) can vary according to cultivar, experimental design, local topography and environmental conditions. The outcrossing rate from one field to another depends also on the size and arrangement of donor and recipient populations and on the ratio between donor and recipient plot size. The outcrossing levels specified in the presented studies are derived mostly from experiments where the recipient field is either surrounding the donor field (continuous design) or is located as a patch at different distances from the donor field (discontinuous design). Reports of gene flow in Brassica napus generally show that the amount of cross-fertilisation decreases as the distance from the pollen source increases. The evidence given in various studies reveals that the bulk of GM cross-fertilisation occurs within the first 10 m of the recipient field. The removal of the first 10 m of a non-transgenic field facing a GM crop might therefore be more efficient for reducing the total level of cross-fertilisation in a recipient sink population than to recommend separation distances. Future experiments should investigate cross-fertilisation with multiple adjacent donor fields at the landscape level under different spatial distributions of rapeseed cultivars and different cropping systems. The level of cross-fertilisation occurring over the whole field is mainly important for co-existence and has not been investigated in agricultural scale experiments until now. Potential problems with herbicide resistant oilseed rape volunteers arising from intraspecific gene flow can be largely solved by the choice of suitable cultivars and herbicides as well as by soil management.
Similar content being viewed by others
References
Beaujean A, Sangwan RS, Hodges M, Sangwan-Norreel BS (1998) Effect of ploidy and homozygosity on transgene expression in primary tobacco transformants and their androgenetic progenies. Mol Gen Genet 260:362–371
Becker HC, Damgaard C, Karlsson B (1992) Environmental variation for outcrossing rate in rapeseed (Brassica napus). Theor Appl Genet 84:303–306
Becker R, Ulrich A, Hedtke C, Honermeier B (2001) Einfluss des Anbaus von transgenem herbizidresistentem Raps auf das Agrar-Ökosystem Bundesgesundheitsbl 44. Jahrgang 2:159–167
Beckie HJ, Warwick SI, Nair H, Séguin-Swartz G (2003) Gene flow in commercial fields of herbicide-resistant canola (Brassica napus). Ecol Appl 13:1276–1294
Brown AP, Brown J, Thill DC, Brammer TA (1996) Gene transfer between canola (Brassica napus) and related weed species. In: Proceedings of the 8th symposium on environmental releases of biotechnology products: risk assessment methods and research progress, Ottawa, Canada
Champolivier J, Gasquez J, Méssean A, Richard-Molard M (1999) Management of transgenic crops within the cropping system. In: Lutman PJW (ed) Gene flow and agriculture–relevance for transgenic crops, British Crop Protection Council, Vol. 72, pp 233–240
Crawford JW, Squire G, Burn D (1999) Modelling spread of herbicide resistance in oilseed rape. In: Gray AJ, Amijee F, Gliddon CJ (eds) Environmental impact of genetically modified crops. Research Report No. 10. DETR, London, pp 97–106
Crawley MJ, Hails RS, Rees M, Kohn D, Buxton J (1993) Ecology of transgenic oilseed rape in natural habitats. Nature 363:620–623
Cresswell JE (1999) The influence of nectar and pollen availability on pollen transfer by individual flowers of oilseed rape (Brassica napus) when pollinated by bumblebees (Bombus lapidarius). J Ecol 87:670–677
Damgaard C, Kjellsson G (2005) Gene flow of oilseed rape (Brassica napus) according to isolation distance and buffer zone. Agric Ecosyst Environ 108:291–301
Daniell H (2002) Molecular strategies for gene containment in transgenic crops. Nat Biotechnol 20:581–586
Devos Y, Reheul D, Schrijver A, Cors F, Moens W (2004) Management of herbicide-tolerant oilseed rape in Europe: a case study on minimizing vertical gene flow. Environ Biosafety Res 3:135–148
Dietz-Pfeilstetter A, Gland-Zwerger A, Garbe V (1999) Potential and valuation of pollen-mediated gene transfer from transgenic oilseed rape. Nachrichtenbl Deut Pflanzenschutzd 51:14–19
Dietz-Pfeilstetter A, Zwerger P (2004) Dispersal of herbicide resistance genes during the large scale cultivation of different transgenic herbicide resistant oilseed rape varieties. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz—J Plant Diseases Protect, Sonderheft XIX:831–838
Downey RK (1999a) Gene flow and rape— the Canadian experience. In: Lutman PJW (ed) Gene flow and agriculture—relevance for transgenic crops, British Crop Protection Council, Vol. 72, pp 109–116
Downey RK (1999b) Risk assessment of outcrossing of transgenic Brassica, with focus on B. rapa and B. napus. In: Proceedings of the 10th international rapeseed Congress, Canberra, Australia, p 6
Eastham K, Sweet J (2002) Genetically modified organisms (GMOs): the significance of gene flow through pollen transfer. Enviromental Issue Report No 28. European Environment Agency, Copenhagen
Eckert JE (1933) The flight range of the honeybee. J Agric Res 47:257–285
Eisikowitch D (1981) Some aspects of pollination of oilseed rape (Brassica napus L.). J agric Sci (Camb) 96:321–326
Emberlin J, Adams-Groom B, Tidmarsh J (1999) The dispersal of maize (Zea mays) pollen. A report based on evidence available from publications and internet sites. A report commissioned by the Soil Association: National Pollen Research Unit, University College Worcester, Worcester, UK
Feldmann S (2000) Begleitforschung zur Freisetzung herbizidresistenter, transgener Rapspflanzen 1995–1999. Ein Beitrag zur biologischen Sicherheitsforschung – Endbericht. In: Niedersächsisches Landesamt für Ökologie (ed) Nachhaltiges Niedersachsen 13 – Dauerhaft umweltgerechte Entwicklung, pp 1–57
Feldmann SD, Brandes S, Pfeilstetter E, Matzk A, Schiemann J (1998) Begleituntersuchungen des Landes Niedersachsen zur Freisetzung transgener, herbizidresistenter Rapspflanzen, Bundesgesundheitsbl 44. Jahrgang 12:536–542
Gliddon C, Boudry P, Walker S (1999) Gene flow—a review of experimental evidence. In: Enviromental impact of genetically modified crops. DETR, London, pp 65–79
Götz R, Ammer F (2000) Ergebnisse der Anwendung von Liberty in transgenem Winterraps in Thüringen. Z. PflKrank. PflSchutz, Sonderh. XVII, pp 397–401
Gruber S, Pekrun C, Claupein W (2004) Population dynamics of volunteer oilseed rape (Brassica napus L.) affected by tillage. Eur J Agron 20:351–361
Hall L, Topinka K, Huffman J, Davis L, Good A (2000) Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Sci 48:688–694
Hayter KE and Cresswell JE (2003) An experimental evaluation of the relative importance of pollination by insects vs. wind in oilseed rape (Brassica napus). In: Boelt B (ed) Proceedings of the 1st European conference on the co-existence of genetically modified crops with conventional and organic crops, Denmark, p 214
Hommel B, Pallutt B (2003) Evaluation of transgenic herbicide-resistant oilseed rape and maize with reference to integrated pest management strategies. In: The BCPC international Congress: Crop Science and Technology 2003, pp 1087–1092
Ingram J (2000) The separation distances required to ensure cross-pollination is below specified limits in non-seed crops of sugar beet, maize and oilseed rape. Plant Var Seeds 13:181–199
James VA, Avart C, Worland B, Snape JW, Vain P (2002) The relationship between homozygous and hemizygous transgene expression levels over generations in populations of transgenic rice plants. Theor Appl Genet 104:553–561
Lavigne C, Klein EK, Valleé P, Pierre J, Godelle B, Renard M (1998) A pollen-dispersal experiment with transgenic oilseed rape. Estimation of the average pollen dispersal of an individual plant within a field. Theor Appl Genet 96:886–896
Morris WF, Kareiva PM, Raymer PL (1994) Do barren zones and pollen traps reduce gene escape from trangenic crops? Ecol Appl 4:157–165
Pierre J (2001) The role of honeybees (Apis mellifera) and other insect pollinators in gene flow between oilseed rape (Brassica napus) and wild radish (Raphanus raphanistrum). Acta Hortic 561:47–51
Rakow G, Woods DL (1987) Outcrossing in rape and mustard under Saskatchewan prairie conditions Can. J. Plant Sci 67:147–151
Ramsay G, Thompson C, Neilson S, Mackay GR (1999) Honeybees as vectors of GM oilseed rape pollen. In: Lutman PJW (ed) Gene flow and agriculture—relevance for transgenic crops, British Crop Protection Council, Vol. 72, pp 209–214
Ramsey G, Thompson C, Squire G (2003) Quantifying landscape-scale gene flow in oilseed rape. Final Report of DEFRA Project RG0216: an experimental and mathematical study of the local and regional scale movement of an oilseed rape transgene
Ranito-Lehtimäki A (1995) Aerobiology of pollen and pollen antigens. In: Cox C and Wathes C (eds) Bioaerosols Handbook, CRC Lewis
Reboud X (2003) Effect of a gap on gene flow between otherwise adjacent transgenic Brassica napus crops. Theor Appl Genet 106:1048–1058
Richards HA, Halfhill MD, Millwood RJ, Stewart CN (2003) Quantitative GFP Fluorescence as an indicator of recombinant protein synthesis in transgenic plants. Plant Cell Rep 22:117–121
Rieger MA, Lamond M, Preston C, Powles SB, Roush RT (2002) Pollen-mediated movement of herbicide resistance between commercial canola fields. Science 296:2386–2388
Salisbury PA (2002) Genetically modified canola in Australia: agronomic and environmental considerations. In: Downey K (ed) Australian Oilseeds Federation, 69 pp
Scheffler JA, Parkinson R, Dale PJ (1993) Frequency and distance of pollen dispersal from transgenic oilseed rape (Brassica napus). Trans Res 2:356–364
Scheffler JA, Parkinson R, Dale PJ (1995) Evaluating the effectiveness of isolation distances for field plots of oilseed rape (Brassica napus) using a herbicide-resistance transgene as a selectable marker. Plant Breed 114:317–321
Scientific Committee on Plants (2001) Opinion of the scientific committee on plants concerning the adventitious presence of GM seeds in conventional seeds. SCP/GMO-SEED-CONT/002-final 13 March 2001
Simpson EC, Norris CE, Law JR, Thomas JE, Sweet JB (1999) Gene flow in genetically modified herbicide tolerant oilseed rape (Brassica napus) in the UK. In: Lutman PJW (ed) Gene flow and agriculture—relevance for transgenic crops, British Crop Protection Council, Vol. 72, pp 75–81
Staniland BK, McVetty PBE, Friesen LF, Yarrow S, Freyssinet G, Freyssinet M (2001) Effectiveness of border areas in confining the spread of transgenic Brassica napus pollen. Can J Plant Sci 80:521–526
Stewart CN Jr, All JN, Raymer PL, Ramachandran S (1997) Increased fitness of transgenic insecticidal rapeseed under insect selection pressure. Mol Ecol 6:773–779
Stringham GR, Downey RK (1982) Effectiveness of isolation distance in seed production of rapeseed (Brassica napus). Agron Abstr 136–137
Tang J, Scarth R, Fristensky B (2003) Effects of genomic position and copy number of Acyl-ACP thioesterase transgenes on the level of the target fatty acids in Brassica napus L. Mol Breed 12:71–81
Thompson CE, Squire G, Mackay GR, Bradshaw, JE Crawford J, Ramsay G (1999) Regional patterns of gene flow and its consequences for GM oilseed rape. In: Lutman PJW (ed) Gene flow and agriculture—relevance for transgenic crops, British Crop Protection Council, Vol. 72, pp 233–240
Timmons AM, O’Brien ET, Charters YM, Dubbels SJ, Wilkinson MJ (1995) Assessing the risks of wind pollination from fields of genetically modified. Brassica napus ssp. Oleifera. Euphytica 85:417–423
Treu R, Emberlin J (2000) Pollen dispersal in the crops Maize (Zea mays), Oil seed rape (Brassica napus ssp oleifera), Potatoes (Solanum tuberosum), Sugar beet (Beta vulgaris ssp. vulgaris) and Wheat (Triticum aestivum). A report for the Soil Association from the National Pollen Research Unit, University College, Worcester, UK
Westcott L, Nelson D (2001) Canola pollination: an update. Bee World 82:115–129
Acknowledgements
We thank Ralf Wilhelm for critically reading the manuscript. One of the authors (A.H.) was supported by an EU grant (project SIGMEA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hüsken, A., Dietz-Pfeilstetter, A. Pollen-mediated intraspecific gene flow from herbicide resistant oilseed rape (Brassica napus L.). Transgenic Res 16, 557–569 (2007). https://doi.org/10.1007/s11248-007-9078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-007-9078-y