Abstract
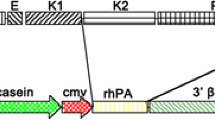
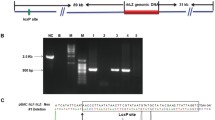
Alkaline phosphatase is a promising therapeutic agent in the Gram-negative bacterial lipopolysaccharide (LPS) mediated acute and chronic diseases. Contrary to other alkaline phosphatase isozymes, purified tissue-nonspecific alkaline phosphatase (TNAP) is not available in large quantities from tissue sources, which would enable to analyse its efficacy in animal sepsis models. Two transgenic rabbit lines were created by pronuclear microinjection with the whey acidic protein promoter-humanTNAP minigene (WAP-hTNAP). Lactating females of both lines produced biologically active human TNAP. As indicated by fractionation of milk samples the recombinant alkaline phosphatase was associated with the membrane of milk fat globules. Alkaline phosphatase enzymatic activity was two orders of magnitude higher compared to normal human serum levels. The demonstration that this TNAP is physiologically active would provide the clue to use transgenic animals as bioreactor for bulk production of the human tissue-nonspecific alkaline phosphatase in milk. This may be a valuable and possibly viable option with important implication in attenuating LPS mediated inflammatory responses.
Similar content being viewed by others
References
Ahn YS, Snow LD (1993) Selective extraction of alkaline phosphatase and 5′-nucleotidase from milk fat globule membranes by a single phase n-butanol procedure. Prep Biochem 23:409–491
Besenfelder U, Strouhal C, Brem G (1998) A method for endoscopic embryo collection and transfer in the rabbit. Zentralbl Veterinarmed A 45:577–579
Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W (2003) Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther 307:737–744
Bleck GT, Jiménez-Flores R, Bremel RD (1995) Abnormal properties of milk from transgenic mice expressing bovine β-casein under control of the bovine α-lactalbumin 5′ flanking region. Int Dairy J 5:619–632
Bősze Zs, Hiripi L, Carnwath JW, Niemann H (2003) The transgenic rabbit as model for human diseases and as a source of biologically active recombinant proteins. Transgenic Res 12:541–553
Burdon T, Wall RJ, Shamay A, Smith GH, Hennighausen L (1991) Over-expression of an endogenous milk protein gene in transgenic mice is associated with impaired mammary alveolar development and a milchlos phenotype. Mech Dev 36:67–74
Castro FO, Limonta J, Rodriguez A, Aguirre A, de la Fuente J, Aguilar A, Ramos B, Hayes O (1999) Transgenic rabbits for the production of biologically active recombinant proteins in the milk. Genet Anal 15:179–187
Chikkappa G (1992) Control of neutrophil alkaline phosphatase synthesis by cytokines in health and diseases. Exp Hematol 20:388–390
Cyboron GW, Wuthier RE (1981) Purification and initial characterization of intrinsic membrane-bound alkaline phosphatase from chicken epiphyseal cartilage. J Biol Chem 256:7262–7268
Devinoy E, Montoliu L, Baranyi M, Thépot D, Hiripi L, Fontaine M-L, Bodrogi L, Bősze Zs (2005) Analysis of the efficiency of the rabbit whey acidic protein gene 5′ flanking region in controlling the expression of homologous and heterologous linked genes. J Dairy Res 72:113–119
DiTullio P, Cheng SH, Marshall J, Gregory RJ, Ebert KM, Meade HM, Smith AE (1992) Production of cystic fibrosis transmembrane conductance regulator in the milk of transgenic mice. Biotechnology 10:74–77
Eisenhaber B, Bork P, Eisenhaber F (2001) Post-translational GPI lipid anchor modification of proteins in kingdoms of life: analysis of protein sequence data from complete genomes. Protein Eng 14:17–25
Fishman WH (1990) Alkaline phosphatase isozymes: recent progress. Clin Biochem 23:99–104
Harris H (1990) The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta 186:133–150
Henthorn PS, Raducha M, Fedde KN, Lafferty MA, Whyte MP (1992) Different missense mutations at the tissue-nonspecific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc Natl Acad Sci USA 89:9924–9928
Hiripi L, Makovics F, Halter R, Baranyi M, Paul D, Carnwath JW, Bősze Z, Niemann H (2003) Expression of active human blood clotting factor VIII in mammary gland of transgenic rabbits. DNA Cell Biol 22:41–45
Hui M, Tenenbaum HC (1998) New face of an old enzyme: alkaline phosphatase may contribute to human tissue aging by inducing tissue hardening and calcification. Anat Rec 253:91–94
Le Du MH, Millan JL (2002) Structural evidence of functional divergence in human alkaline phosphatases. J Biol Chem 277:49808–49814
Magnusson P, Arlesting L, Paus E, Di Mauro S, Testa MP, Stigbrand T, Farley JR, Nustad K, Millan JL (2002) Monoclonal antibodies against tissue-nonspecific alkaline phosphatase. Report of the ISOBM TD9 workshop. Tumour Biol 23:228–248
Massoud M, Attal J, Thepot D, Pointu H, Stinnakre MG, Theron MC, Lopez C, Houdebine LM (1996) The deleterious effects of human erythropoietin gene driven by the rabbit whey acidic protein gene promoter in transgenic rabbits. Reprod Nutr Dev 36:555–563
Poelstra K, Bakker WW, Klok PA, Hardonk MJ, Meijer DK (1997a) A physiologic function for alkaline phosphatase: endotoxin detoxification. Lab Invest 76:319–327
Poelstra K, Bakker WW, Klok PA, Kamps JA, Hardonk MJ, Meijer DK (1997b) Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol 151:1163–1169
Shamay A, Solinas S, Pursel VG, McKnight RA, Alexander L, Beattie C, Hennighausen L, Wall RJ (1991) Production of the mouse whey acidic protein in transgenic pigs during lactation. J Anim Sci 69:4552–4562
Shamay A, Pursel VG, Wilkinson E, Wall RJ, Hennighausen L (1992) Expression of the whey acidic protein in transgenic pigs impairs mammary development. Transgenic Res 1:124–132
Stromme JH, Rustad P, Steensland H, Theodorsen L, Urdal P (2004) Reference intervals for eight enzymes in blood of adult females and males measured in accordance with the International Federation of Clinical Chemistry reference system at 37°C: part of the Nordic Reference Interval Project. Scand J Clin Lab Invest 64:371–384
Tietz NW, Shuey DF (1986) Reference intervals for alkaline phosphatase activity determined by the IFCC␣and AACC reference methods. Clin Chem 32:1593–1594
van Veen SQ, van Vliet AK, Wulferink M, Brands R, Boermeester MA, van Gulik TM (2005) Bovine intestinal alkaline phosphatase attenuates the inflammatory response in secondary peritonitis in mice. Infect Immun 73:4309–4314
Waymire KG, Mahuren JD, Jaje JM, Guilarte TR, Coburn SP, MacGregor GR (1995) Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet 11:45–51
Xu Q, Lu Z, Zhang X (2002) A novel role of alkaline phosphatase in protection from immunological liver injury in mice. Liver 22:8–14
Acknowledgments
The authors wish to specially acknowledge the valuable advices of Michelle Ollivier-Bousquet at INRA, France in evaluating the histological data. We are indebted to Heiner Niemann (FAL, Germany) for critical reading of the manuscript. This work was supported by the Grants: NWO-OTKA N37293, OTKA T04934, GVOP-AKF 71/2004 and EU-COST B20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bodrogi, L., Brands, R., Raaben, W. et al. High Level Expression of Tissue-Nonspecific Alkaline Phosphatase in the Milk of Transgenic Rabbits. Transgenic Res 15, 627–636 (2006). https://doi.org/10.1007/s11248-006-9015-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-006-9015-5