Abstract
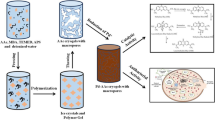
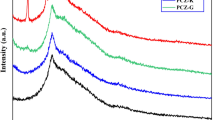
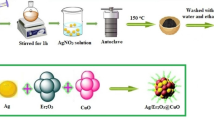
Geopolymers are a type of inorganic polymer that typically have a monochromatic appearance. This study investigates the formation of artificial chrysocolla by immersing these materials in Cu(II) ion solutions. The resulting synthetic chrysocolla possesses magnetic properties and characteristic silicate bands in its structure, indicating geopolymerization and a similarity to natural chrysocolla. Furthermore, the inclusion of copper and zinc ferrites in the nano-modified geopolymer matrix enhances its properties and potential antiviral properties. The antiviral test results show that synthetic chrysocolla based on nano-modified geopolymers has a mild antimicrobial effect. However, more testing is needed to determine its effectiveness against bacteriophage, as bacterial growth was observed in the area where the nano-modified geopolymer bacteriophage blend was applied. In addition to its potential antiviral properties, synthetic chrysocolla's porous structure allows it to act as an adsorbent, making it useful in the development of antiviral filters. Artificial chrysocolla can be obtained through a geopolymeric matrix by immersing it in a solution of Cu(II) ions, which can occur by ion exchange of the ions present in the aluminosilicate structure with Cu(II). Neutralization of the geopolymer matrix by washing with distilled water is required prior to immersion to ensure proper formation of chrysocolla and not copper(II) hydroxide on the geopolymer surface. Overall, this study provides evidence for the successful preparation of nano-modified synthetic chrysocolla from geopolymers and ferrites, with properties and chemical composition similar to those of natural chrysocolla. The presence of copper ions in its composition and the incorporation of copper and zinc ferrites can contribute to its use as a resource to combat viral pathogens.

















Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- cm:
-
Centimeter
- Co:
-
Cobalt
- COVID:
-
Coronavirus disease
- Cu:
-
Copper
- Cu(I):
-
Copper (I)
- Cu(II):
-
Copper (II)
- DOCTYPE:
-
Document Type
- dox:
-
Doxorubicin
- E. coli :
-
Escherichia coli
- EAB:
-
Equivalent absorption bandwidth
- eab:
-
Equivalent absorption bandwidth
- emi se:
-
Electromagnetic interference shielding effectiveness
- emw:
-
Electromagnetic wave
- Fe:
-
Iron
- FWHM:
-
Full-width at the half-maximum
- GHz:
-
Gigahertz
- hco3:
-
Bicarbonate
- Hz:
-
Hertz
- mg g:
-
Milligram per gram
- Mn:
-
Manganese
- Ni:
-
Nickel
- pb2:
-
Lead (II)
- RIS:
-
Research Information Systems
- rlmin:
-
Minimum reflection loss
- RMSE:
-
Root mean square error
- ROS:
-
Reactive Oxygen Species
- semedx:
-
Scanning electron microscopy-energy dispersive X-ray spectroscopy
- VOSviewer:
-
Visualization of Similarities Viewer
- w h kg:
-
Watt-hours per kilogram
- Zn(II):
-
Zinc (II)
References
Davidovits J (1989) Geopolymers and geopolymeric materials. J Therm Anal 35:429–441. https://doi.org/10.1007/BF01904446
Davidovits J (2020) Geopolymer chemistry and applications. Saint-Quentin
Davidovits J (2017) Geopolymers: ceramic-like inorganic polymers. J Ceram Sci Technol 8:335–350. https://doi.org/10.4416/JCST2017-00038
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, van Deventer JSJ (2007) Geopolymer technology: the current state of the art. J Mater Sci 42:2917–2933. https://doi.org/10.1007/s10853-006-0637-z
Tchakoute Kouamo H, Elimbi A, Mbey JA, Ngally Sabouang CJ, Njopwouo D (2012) The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: a comparative study. Const Build Mater 35:960–969. https://doi.org/10.1016/j.conbuildmat.2012.04.023
Wang H, Li H, Yan F (2005) Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf A Physicochem Eng Asp 268:1–6. https://doi.org/10.1016/j.colsurfa.2005.01.016
Maranhão FS, Gomes F, Thode S, Das DB, Pereira E, Lima N, Carvalho F, Aboelkheir M, Costa V, Pal K (2021) Oil spill sorber based on extrinsically magnetizable porous geopolymer. Materials 14:1–11. https://doi.org/10.3390/ma14195641
Bai C, Colombo P (2018) Processing, properties and applications of highly porous geopolymers: a review. Ceram Int 44:16103–16118. https://doi.org/10.1016/j.ceramint.2018.05.219
Cheng TW, Lee ML, Ko MS, Ueng TH, Yang SF (2012) The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl Clay Sci 56:90–96. https://doi.org/10.1016/j.clay.2011.11.027
Takeda H, Hashimoto S, Honda S, Iwamoto Y (2014) The coloring of geopolymers by the addition of copper compounds. Ceram Int 40:6503–6507. https://doi.org/10.1016/j.ceramint.2013.11.103
Hashimoto S, Takeda H, Honda S, Iwamoto Y (2015) Novel coloring of geopolymer products using a copper solution immersion method. Const Build Mater 88:143–148. https://doi.org/10.1016/j.conbuildmat.2015.04.016
Hashimoto S, Machino T, Takeda H, Daiko Y, Honda S, Iwamoto Y (2015) Antimicrobial activity of geopolymers ion-exchanged with copper ions. Ceram Int 41:13788–13792. https://doi.org/10.1016/j.ceramint.2015.08.061
Konieczny J, Rdzawski Z (2012) Antibacterial properties of copper and its alloys. Arch Mater Sci Eng 56:53–60
Sharmin S, Rahaman MdM, Sarkar C, Atolani O, Islam MT, Adeyemi OS (2021) Nanoparticles as antimicrobial and antiviral agents: a literature-based perspective study. Heliyon 7:e06456. https://doi.org/10.1016/j.heliyon.2021.e06456
Vincent M, Duval RE, Hartemann P, Engels-Deutsch M (2018) Contact killing and antimicrobial properties of copper. J Appl Microbiol 124:1032–1046. https://doi.org/10.1111/jam.13681
Govind V, Bharadwaj S, Sai Ganesh MR, Vishnu J, Shankar KV, Shankar B, Rajesh R (2021) Antiviral properties of copper and its alloys to inactivate COVID-19 virus: a review. Biometals 34:1217–1235. https://doi.org/10.1007/s10534-021-00339-4
Noyce JO, Michels H, Keevil CW (2007) Inactivation of Influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol 73:2748–2750. https://doi.org/10.1128/AEM.01139-06
Merkl P, Long S, McInerney GM, Sotiriou GA (2021) Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against sars-cov-2. Nanomater 11:1312. https://doi.org/10.3390/nano11051312
Bhattacharjee S, Bahl P, Macintyre CR, Heslop D (2022) Face masks and respirators: towards sustainable materials and technologies to overcome the shortcomings and challenges. Nano Sel 3:1355–1381. https://doi.org/10.1002/nano.202200101
Alavi M, Kamarasu P, Julian D, Moore MD (2018) Metal and metal oxide-based antiviral nanoparticles : properties, mechanisms of action , and applications. Adv Colloid Interface Sci 306:102726. https://doi.org/10.1016/j.cis.2022.102726
Siddiqi KS, ur Rahman A, Tajuddin, Husen A (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nano Res Lett 13:141. https://doi.org/10.1186/s11671-018-2532-3
Fadl FA, Abu aita NA, Abdelaziz MA, Mohamed AH (2021) In vitro assessment of antimicrobial activity of zinc oxide nanoparticles against pathogenic Streptococcus parauberis. AAVS 9:913–918. https://doi.org/10.17582/journal.aavs/2021/9.6.913.918
Edalatpanah Y, Rahedan F, Rostami M, Rezaei H, Sanaeiyan K, HosseiniAlvand P (2014) Investigation of antibacterial activity of ZnO nanoparticles suspension containing citric acid against Salmonella typhimurium in mango and carrot juice. JBTW 3:38–43. https://doi.org/10.15412/J.JBTW.01030203
Gupta J, Irfan M, Ramgir N, Muthe KP, Debnath AK, Ansari S, Gandhi J, Ranjith-Kumar CT, Surjit M (2022) Antiviral activity of zinc oxide nanoparticles and tetrapods against the Hepatitis E and Hepatitis C viruses. Front Microbiol 13:881595. https://doi.org/10.3389/fmicb.2022.881595
Kefeni KK, Msagati TAM, Mamba BB (2017) Ferrite nanoparticles: synthesis, characterisation and applications in electronic device. Mater Sci Eng B 215:37–55. https://doi.org/10.1016/j.mseb.2016.11.002
Chaibakhsh N, Moradi-Shoeili Z (2019) Enzyme mimetic activities of spinel substituted nanoferrites (MFe2O4): a review of synthesis, mechanism and potential applications. Mater Sci Eng C 99:1424–1447. https://doi.org/10.1016/j.msec.2019.02.086
Masunga N, Mmelesi OK, Kefeni KK, Mamba BB (2019) Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: Review. J Environ Chem Eng 7:103179. https://doi.org/10.1016/j.jece.2019.103179
Weiss C, Carriere M, Fusco L, Fusco L, Capua I, Regla-Nava JA, Pasquali M, Scott JA, Vitale F, Unal M, Mattevi C, Bedognetti D, Merkoçi A, Tasciotti E, Yilmazer A, Gogotsi Y, Stellaci F, Delogu L (2020) Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 14:6383–6406. https://doi.org/10.1021/acsnano.0c03697
Maranhão FDS (2022) The use of magnetically modified geopolymers produced in heterogeneous medium and their application in environmental remediation. Instituto de Macromoléculas Professora Eloisa Mano. https://buscaintegrada.ufrj.br/Record/aleph-UFR01-000926459
Tan TH, Mo KH, Lai SH, Ling TC (2022) Investigation on the copper ion removal potential of a facile-fabricated foamed geopolymer sphere for wastewater remediation. Clean Mater 4:100088. https://doi.org/10.1016/j.clema.2022.100088
Souza FG, Ferreira AC, Varela A, Oliveira GE, Machado F, Pereira ED, Fernandes E, Pinto JC, Nele M (2013) Methodology for determination of magnetic force of polymeric nanocomposites. Polym Test 32:1466–1471. https://doi.org/10.1016/j.polymertesting.2013.09.018
Millard AD (2009). In: Clokie MRJ, Kropinski AM (eds) Isolation of cyanophages from aquatic environments. Humana Press, New Jersey
Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP (2009). In: Clokie MRJ, Kropinski AM (eds) Enumeration of bacteriophages by double Agar overlay plaque assay. Humana Press, New Jersey
Khawaja KA, Abbas Z, Rehman SU (2016) Isolation and characterization of lytic phages TSE1-3 against Enterobacter cloacae. Open Life Sci 11:287–292. https://doi.org/10.1515/biol-2016-0038
Ly-Chatain MH, Moussaoui S, Vera A, Rigobello V, Demarigny Y (2013) Antiviral effect of cationic compounds on bacteriophages. Front Microbiol 4:1–6. https://doi.org/10.3389/fmicb.2013.00046
Manohar P, Tamhankar AJ, Lundborg CS, Nachimuthu R (2019) Therapeutic characterization and efficacy of bacteriophage cocktails infecting Escherichia coli, Klebsiella pneumoniae, and Enterobacter Species. Front Microbiol 10:574. https://doi.org/10.3389/fmicb.2019.00574
Gajic I, Kabic J, Kekic D, Jovicevic M, Milenkovic M, Mitic Culafic D, Trudic A, Ranin L, Opavski N (2022) Antimicrobial susceptibility testing: a comprehensive review of currently used methods. Antibiotics 11:427. https://doi.org/10.3390/antibiotics11040427
Casbeer E, Sharma VK, Li XZ (2012) Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep Purif Technol 87:1–14. https://doi.org/10.1016/j.seppur.2011.11.034
Saldaña M, Gálvez E, Robles P, Castillo J, Toro N (2022) Copper mineral leaching mathematical models—a review. Materials 15:1757. https://doi.org/10.3390/ma15051757
Liu Y, Ai K, Lu L (2014) Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev 114:5057–5115. https://doi.org/10.1021/cr400407a
Reizabal A, Costa CM, Pérez-Álvarez L, Vilas-Vilela JL, Lanceros-Méndez S (2023) Silk fibroin as sustainable advanced material: material properties and characteristics, processing, and applications. Adv Funct Mater 33:2210764. https://doi.org/10.1002/adfm.202210764
Žalnėravičius R, Pakštas V, Grincienė G, Klimas V, Paškevičius A, Timmo K, Kauk-Kuusik M, Franckevičius M, Niaura G, Talaikis G, Jagminas A, Ramanavičius A (2023) Antimicrobial particles based on Cu2ZnSnS4 monograins. Colloids Surf B Biointerface 225:113275. https://doi.org/10.1016/j.colsurfb.2023.113275
Somvanshi SB, Jadhav SA, Gawali SS, Zakde K, Jadhav KM (2023) Core-shell structured superparamagnetic Zn–Mg ferrite nanoparticles for magnetic hyperthermia applications. J Alloys Compd 947:169574. https://doi.org/10.1016/j.jallcom.2023.169574
Camacho-González MA, Quezada-Cruz M, Cerón-Montes GI, Ramírez-Ayala MF, Hernández-Cruz LE, Garrido-Hernández A (2019) Synthesis and characterization of magnetic zinc-copper ferrites: antibacterial activity, photodegradation study and heavy metals removal evaluation. Mater Chem Phys 236:121808. https://doi.org/10.1016/j.matchemphys.2019.121808
Waldron RD (1955) Infrared spectra of ferrites. Phys Rev 99:1727–1735. https://doi.org/10.1103/PhysRev.99.1727
Petrila I, Tudorache F (2021) Effects of sintering temperature on the microstructure, electrical and magnetic characteristics of copper–zinc spinel ferrite with possibility use as humidity sensors. Sensors Actuators A Phys 332:113060. https://doi.org/10.1016/j.sna.2021.113060
Subha A, Shalini MG, Sahu BN, Rout S, Sahoo SC (2022) Role of surface defects and anisotropy variation on magnetic properties of copper ferrite nanoparticles prepared by co-precipitation method. Mater Chem Phys 286:126212. https://doi.org/10.1016/j.matchemphys.2022.126212
Rossatto DL, Netto MS, Jahn SL, Mallmann ES, Dotto GL, Foletto EL (2020) Highly efficient adsorption performance of a novel magnetic geopolymer/Fe3O4 composite towards removal of aqueous acid green 16 dye. J Environ Chem Eng 8:103804. https://doi.org/10.1016/j.jece.2020.103804
Shen P, Liu D, Xu X, Jia X, Zhang X, Liu D, Liu R (2018) Effect of ethylene diamine phosphate on the sulfidization flotation of chrysocolla. Minerals 8:216. https://doi.org/10.3390/min8050216
Park S, Pour-Ghaz M (2018) What is the role of water in the geopolymerization of metakaolin? Const Build Mater 182:360–370. https://doi.org/10.1016/j.conbuildmat.2018.06.073
Ferreira LP, Moreira AN, Delazare T, Oliveira GE, Souza FG (2012) Petroleum absorbers based on CNSL, furfural and lignin—the effect of the chemical similarity on the interactions among petroleum and bioresins. Macromol Symp 319:210–221. https://doi.org/10.1002/masy.201100145
Ferreira LP, da Cunha BP, Kuster RM, Pinto JC, Souza MN, de Souza FG (2017) Synthesis and chemical modification of poly(butylene succinate) with rutin useful to the release of silybin. Ind Crop Prod 97:599–611. https://doi.org/10.1016/j.indcrop.2016.12.064
Nayak H (2016) Effect of catalytic activities of mixed nano ferrites of zinc and copper on decomposition kinetics of lanthanum oxalate hydrate. Trans Nonferrous Met Soc China 26:767–774. https://doi.org/10.1016/S1003-6326(16)64135-3
Vissurkhanova YaA, Ivanova NM, Soboleva YeA, Muldakhmetov ZM, Abulyaissova LK, Minaev BF (2023) Fe–Cu composites preparation using Cu–Zn ferrite and their electrocatalytic application. Mater Lett 333:133521. https://doi.org/10.1016/j.matlet.2022.133521
Mugnaini S, Bagnoli A, Bensi P, Droghini F, Scala A, Guasparri G (2006) Thirteenth century wall paintings under the Siena Cathedral (Italy). Mineralogical and petrographic study of materials, painting techniques and state of conservation. J Cult Herit 7:171–185. https://doi.org/10.1016/j.culher.2006.04.002
Morel M, Martínez F, Mosquera E (2013) Synthesis and characterization of magnetite nanoparticles from mineral magnetite. J Magn Magn Mater 343:76–81. https://doi.org/10.1016/j.jmmm.2013.04.075
Balaraman S, Iruson B, Krishnmoorthy S, Elayaperumal M (2021). In: Khan M (ed) The presented study of Zn–Cu ferrites for their application in “Photocatalytic Activities, IntechOpen. https://www.intechopen.com/chapters/78001. Accessed 12 Apr 2023
Nadeem K, Zeb F, Azeem Abid M, Mumtaz M, Anis ur Rehman M (2014) Effect of amorphous silica matrix on structural, magnetic, and dielectric properties of cobalt ferrite/silica nanocomposites. J Non Cryst Sol 400:45–50. https://doi.org/10.1016/j.jnoncrysol.2014.05.004
Das Jana I, Kumbhakar P, Banerjee S, Gowda CC, Kedia N, Kuila SK, Banerjee S, Das Chandra N, Das Kumar A, Manna I, Tiwary CS, Mondal A (2021) Copper nanoparticle-graphene composite-based transparent surface coating with antiviral activity against influenza virus. ACS Appl Nano Mater 4:352–362. https://doi.org/10.1021/acsanm.0c02713
Acknowledgements
We thank the Macromolecules Institute Professor Eloisa Mano (IMA) for all the support. We also thank the Center of Mineralogy (CETEM), for the SEM analyses, conducted by Antonieta Middea, the Marine Research Institute (IPqM) by the synthesis of magnetic nanoparticles conducted by Roberto Costa Lima and the Faculty of Pharmacy by the antiviral efficiency tests conducted by Sérgio Lisboa Machado. Besides, this work was supported by Agência Nacional de Petróleo (PRH 16.1), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq BRICS-STI-5 440090/2022-9 and PQ-2022 302508/2022-8), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES–Finance Code 001), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ E-26/210.800/2021 (Energy), E-26/211.122/2021 (COVID), E-26/210.511/2021 (ConBraPA2022), E-26/201.154/2021 (CNE), and E-26/210.080/2023 (Thematic)).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, G.B., da Silveira Maranhão, F., de Souza, F.G. et al. Artificial Chrysocolla with Catalyst Nanomodified with Copper and Zinc. Top Catal 67, 86–102 (2024). https://doi.org/10.1007/s11244-023-01842-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01842-3