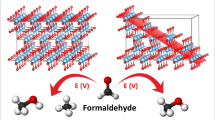
Abstract
Understanding the adsorption and reactivity of carboxylic acids on oxide surfaces is of great interest in catalysis for biomass upgrading via ketonization, a carbon–carbon coupling reaction. Herein, we investigate the adsorption and reaction of acetic acid on anatase TiO2(101) using scanning tunneling microscopy, infrared spectroscopy, temperature programmed reaction, and density functional theory calculations. We demonstrate the adsorption of acetic acid can form two intermediates: (1) dissociated, bidentate acetate with an associated bridging hydroxyl, and (2) molecular, monodentate acetic acid. The coexistence of ordered phases with increasing monolayer (ML) saturation coverages consisting of (1) pure acetate (0.5 ML), (2) mixed acetate/acetic acid (0.67 ML), (3) mixed acetate/acetic acid (1.0 ML) and (4) pure acetic acid demonstrates similar energetics for both acetate and acetic acid species. Under ultra-high vacuum conditions, the presence of both monodentate acetic acid and bidentate acetate was observed below room temperature, while solely bidentate acetate was observed up to 575 K. The deprotonation of acetic acid produces water at 280 K, while the thermal decomposition of bidentate acetate produces ketene and acetic acid at 645 K. This model study provides detailed insight into the stability and reactivity of carboxylic acid surface-bound intermediates, which could participate during ketonization reactions for biomass upgrading.









Similar content being viewed by others
Data Availability
All experimental and theoretical data are provided in the manuscript and the supporting information files.
Change history
29 September 2023
ESM has been updated
References
Pham TN, Sooknoi T, Crossley SP, Resasco DE (2013) Ketonization of carboxylic acids: mechanisms, catalysts, and implications for biomass conversion. ACS Catal 3:2456–2473. https://doi.org/10.1021/cs400501h
Boekaerts B, Sels BF (2021) Catalytic advancements in carboxylic acid ketonization and its perspectives on biomass valorisation. Appl Catal B Environ 283:119607. https://doi.org/10.1016/j.apcatb.2020.119607
Climent MJ, Corma A, Iborra S (2014) Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem 16:516–547. https://doi.org/10.1039/c3gc41492b
Pacchioni G (2014) Ketonization of carboxylic acids in biomass conversion over TiO2 and ZrO2 surfaces: a DFT perspective. ACS Catal 4:2874–2888. https://doi.org/10.1021/cs500791w
Hasan MA, Zaki MI, Pasupulety L (2003) Oxide-catalyzed conversion of acetic acid into acetone: an FTIR spectroscopic investigation. Appl Catal A Gen 243:81–92. https://doi.org/10.1016/S0926-860X(02)00539-2
Pestman R, van Duijne A, Pieterse JAZ, Ponec V (1995) The formation of ketones and aldehydes from carboxylic acids, structure-activity relationship for two competitive reactions. J Mol Catal A Chem 103:175–180. https://doi.org/10.1016/1381-1169(95)00138-7
Pestman R, Koster RM, van Duijne A et al (1997) Reactions of carboxylic acids on oxides. J Catal 168:265–272. https://doi.org/10.1006/jcat.1997.1624
Pham TN, Shi D, Resasco DE (2014) Kinetics and mechanism of ketonization of acetic acid on Ru/TiO2 catalyst. Top Catal 57:706–714. https://doi.org/10.1007/s11244-013-0227-7
Wang S, Iglesia E (2017) Experimental and theoretical assessment of the mechanism and site requirements for ketonization of carboxylic acids on oxides. J Catal 345:183–206. https://doi.org/10.1016/j.jcat.2016.11.006
Kim KS, Barteau MA (1990) Structure and composition requirements for deoxygenation, dehydration, and ketonization reactions of carboxylic acids on TiO2(001) single-crystal surfaces. J Catal 125:353–375. https://doi.org/10.1016/0021-9517(90)90309-8
Tanner RE, Liang Y, Altman EI (2002) Structure and chemical reactivity of adsorbed carboxylic acids on anatase TiO2(001). Surf Sci 506:251–271. https://doi.org/10.1016/S0039-6028(02)01388-2
Martinez R, Huff MCC, Barteau MAA (2004) Ketonization of acetic acid on titania-functionalized silica monoliths. J Catal 222:404–409. https://doi.org/10.1016/j.jcat.2003.12.002
Pham TN, Shi D, Resasco DE (2014) Reaction kinetics and mechanism of ketonization of aliphatic carboxylic acids with different carbon chain lengths over Ru/TiO2 catalyst. J Catal 314:149–158. https://doi.org/10.1016/j.jcat.2014.04.008
White JM, Szanyi J, Henderson MA (2004) Thermal chemistry of trimethyl acetic acid on TiO2(110). J Phys Chem B 108:3592–3602. https://doi.org/10.1021/jp036713w
Grinter DC, Nicotra M, Thornton G (2012) Acetic acid adsorption on anatase TiO2(101). J Phys Chem C 116:11643–11651. https://doi.org/10.1021/jp303514g
Petrik NG, Wang Y, Wen B et al (2021) Conversion of formic acid on single- and nano-crystalline anatase TiO2(101). J Phys Chem C 125:7686–7700. https://doi.org/10.1021/acs.jpcc.1c00571
Wang Y, Wen B, Dahal A et al (2020) Binding of formic acid on anatase TiO2(101). J Phys Chem C 124:20228–20239. https://doi.org/10.1021/acs.jpcc.0c06031
Diebold U (2003) The surface science of titanium dioxide. Surf Sci Rep 48:53–229. https://doi.org/10.1016/s0167-5729(02)00100-0
Ma R, O’Connor CR, Collinge G et al (2023) The role of surface hydroxyls in the mobility of carboxylates on surfaces: dynamics of acetate on anatase TiO2(101). J Phys Chem Lett 14:2542–2550. https://doi.org/10.1021/acs.jpclett.3c00175
Murphy DM, Koop T (2005) Review of the vapour pressures of ice and supercooled water for atmospheric applications. Q J R Meteorol Soc 131:1539–1565. https://doi.org/10.1256/qj.04.94
Smith RS, Matthiesen J, Knox J, Kay BD (2011) Crystallization kinetics and excess free energy of H2O and D2O nanoscale films of amorphous solid water. J Phys Chem A 115:5908–5917. https://doi.org/10.1021/jp110297q
Smith RS, Kay BD (2019) Desorption kinetics of carbon dioxide from a graphene-covered Pt(111) surface. J Phys Chem A 123:3248–3254. https://doi.org/10.1021/acs.jpca.9b00674
Setvín M, Daniel B, Mansfeldova V et al (2014) Surface preparation of TiO2 anatase (101): pitfalls and how to avoid them. Surf Sci 626:61–67. https://doi.org/10.1016/j.susc.2014.04.001
Herman GS, Dohnálek Z, Ruzycki N, Diebold U (2003) Experimental investigation of the interaction of water and methanol with anatase-TiO2(101). J Phys Chem B 107:2788–2795. https://doi.org/10.1021/jp0275544
Scoles G (1988) Atomic and molecular beam methods:, vol 1. Oxford University Press, New York
Ramsey NF (1985) Molecular beams. Oxford University Press, Oxford
Xu B, Madix RJ, Friend CM (2010) Achieving optimum selectivity in oxygen assisted alcohol cross-coupling on gold. J Am Chem Soc 132:16571–16580. https://doi.org/10.1021/ja106706v
O’Connor CR, Hiebel F, Chen W et al (2018) Identifying key descriptors in surface binding: interplay of surface anchoring and intermolecular interactions for carboxylates on Au(110). Chem Sci 9:3759–3766. https://doi.org/10.1039/C7SC05313D
Możejko P (2007) Calculations of electron impact ionization cross section for simple biomolecules: formic and acetic acids. Eur Phys J Spec Top 144:233–237. https://doi.org/10.1140/epjst/e2007-00133-8
Bull JN, Harland PW (2008) Absolute electron impact ionization cross-sections and polarisability volumes for C2 to C4 aldehydes, C4 and C6 symmetric ethers and C3 to C6 ketones. Int J Mass Spectrom 273:53–57. https://doi.org/10.1016/j.ijms.2008.03.003
Kim Y-KK, Irikura KK, Rudd ME, et al (2004) Electron-impact cross sections for ionization and excitation database. NIST Stand. Ref. Database 107
Krems M, Zirbel J, Thomason M, DuBois RD (2005) Channel electron multiplier and channelplate efficiencies for detecting positive ions. Rev Sci Instrum. https://doi.org/10.1063/1.2052052
Ko EI, Benziger JB, Madix RJ (1980) Reactions of methanol on W(100) and W(100)-(5×1)C surfaces. J Catal 62:264–274. https://doi.org/10.1016/0021-9517(80)90454-6
Straub HC, Renault P, Lindsay BG et al (1996) Absolute partial cross sections for electron-impact ionization of H2, N2, and O2 from threshold to 1000 eV. Phys Rev A 54:2146–2153. https://doi.org/10.1103/PhysRevA.54.2146
Chabal YJ (1988) Surface infrared spectroscopy. Surf Sci Rep 8:211–357. https://doi.org/10.1016/0167-5729(88)90011-8
Chabal YJ (1987) Vibrational properties at semiconductor surfaces and interfaces. In: Le Lay G, Derrien J, Boccara N (eds) Semiconductor interfaces: formation and properties. Springer, Berlin, Heidelberg
Kimmel GA, Baer M, Petrik NG et al (2012) Polarization- and azimuth-resolved infrared spectroscopy of water on TiO2(110): anisotropy and the hydrogen-bonding network. J Phys Chem Lett 3:778–784. https://doi.org/10.1021/jz3001079
Hutter J, Iannuzzi M, Schiffmann F, VandeVondele J (2014) CP2K: atomistic simulations of condensed matter systems. Wiley Interdiscip Rev Comput Mol Sci 4:15–25. https://doi.org/10.1002/wcms.1159
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. https://doi.org/10.1103/PhysRev.140.A1133
Goedecker S, Teter M, Hutter J (1996) Separable dual-space Gaussian pseudopotentials. Phys Rev B 54:1703–1710. https://doi.org/10.1103/PhysRevB.54.1703
VandeVondele J, Hutter J (2007) Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J Chem Phys. https://doi.org/10.1063/1.2770708
Dudarev S, Botton G (1998) Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys Rev B—Condens Matter Mater Phys 57:1505–1509. https://doi.org/10.1103/PhysRevB.57.1505
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions: II—overlap populations, bond orders, and covalent bond energies. J Chem Phys 23:1841–1846. https://doi.org/10.1063/1.1740589
Doudin N, Collinge G, Gurunathan PK et al (2021) Creating self-assembled arrays of mono-oxo (MoO3)1 species on TiO2(101) via deposition and decomposition of (MoO3)n oligomers. Proc Natl Acad Sci 118:e2017703118. https://doi.org/10.1073/pnas.2017703118
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys. Doi 10(1063/1):3382344
Doudin N, Collinge G, Persaud RR et al (2021) Binding and stability of MgO monomers on anatase TiO2(101). J Chem Phys 154:204703. https://doi.org/10.1063/5.0047521
Spreafico C, Schiffmann F, VandeVondele J (2014) Structure and mobility of acetic acid at the anatase (101)/acetonitrile interface. J Phys Chem C 118:6251–6260. https://doi.org/10.1021/jp4117563
He Y, Tilocca A, Dulub O et al (2009) Local ordering and electronic signatures of submonolayer water on anatase TiO2(101). Nat Mater 8:585–589. https://doi.org/10.1038/nmat2466
Zhao Z, Li Z, Zou Z (2012) Structure and properties of water on the anatase TiO2(101) surface: from single-molecule adsorption to interface formation. J Phys Chem C 116:11054–11061. https://doi.org/10.1021/jp301468c
Petersen T, Klüner T (2020) Water adsorption on ideal anatase-TiO2(101)—an embedded cluster model for accurate adsorption energetics and excited state properties. Zeitschrift für Phys Chemie 234:813–834. https://doi.org/10.1515/zpch-2019-1425
Tolba SA, Sharafeldin I, Allam NK (2020) Comparison between hydrogen production via H2S and H2O splitting on transition metal-doped TiO2(101) surfaces as potential photoelectrodes. Int J Hydrogen Energy 45:26758–26769. https://doi.org/10.1016/j.ijhydene.2020.07.077
Chao J, Zwolinski BJ (1978) Ideal gas thermodynamic properties of methanoic and ethanoic acids. J Phys Chem Ref Data 7:363–377. https://doi.org/10.1063/1.555571
Altman EI, Tanner RE (2003) Using scanning tunneling microscopy to characterize adsorbates and reactive intermediates on transition metal oxide surfaces. Catal Today 85:101–111. https://doi.org/10.1016/S0920-5861(03)00379-1
Xu C, Koel BE (1995) Adsorption and reaction of CH3COOH and CD3COOD on the MgO(100) surface: a Fourier transform infrared and temperature programmed desorption study. J Chem Phys 102:8158–8166. https://doi.org/10.1063/1.469227
González F, Munuera G, Prieto JA (1978) Mechanism of ketonization of acetic acid on anatase TiO2 surfaces. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 74:1517. https://doi.org/10.1039/f19787401517
Tao J, Luttrell T, Bylsma J, Batzill M (2011) Adsorption of acetic acid on rutile TiO2 (110) vs (011)-2 × 1 surfaces. J Phys Chem C 115:3434–3442. https://doi.org/10.1021/jp111270x
Rajadurai S (1994) Pathways for carboxylic acid decomposition on transition metal oxides. Catal Rev 36:385–403. https://doi.org/10.1080/01614949408009466
Stubenrauch J, Brosha E, Vohs JM (1996) Reaction of carboxylic acids on CeO2(111) and CeO2(100). Catal Today 28:431–441. https://doi.org/10.1016/S0920-5861(96)00251-9
Bowker M, Houghton H, Waugh KC (1983) The interaction of acetaldehyde and acetic acid with the ZnO surface. J Catal 79:431–444. https://doi.org/10.1016/0021-9517(83)90336-6
Deskins NA, Kimmel GA, Petrik NG (2020) Observation of molecular hydrogen produced from bridging hydroxyls on anatase TiO2(101). J Phys Chem Lett 2:9289–9297. https://doi.org/10.1021/acs.jpclett.0c02735
Hugenschmidt MB, Gamble L, Campbell CT (1994) The interaction of H2O with a TiO2(110) surface. Surf Sci 302:329–340. https://doi.org/10.1016/0039-6028(94)90837-0
Henderson MA (1996) Structural sensitivity in the dissociation of water on TiO2 single-crystal surfaces. Langmuir 12:5093–5098. https://doi.org/10.1021/la960360t
Perkins CL, Henderson MA, September RV (2001) Photodesorption and trapping of molecular oxygen at the TiO2(110)—water ice interface. J Phys Chem B 105:3856–3863. https://doi.org/10.1021/jp0031799
Lane CD, Petrik NG, Orlando TM, Kimmel GA (2007) Electron-stimulated oxidation of thin water films adsorbed on TiO2(110). J Phys Chem C 111:16319–16329. https://doi.org/10.1021/jp072479o
Du Y, Petrik NG, Deskins NA et al (2012) Hydrogen reactivity on highly-hydroxylated TiO2(110) surfaces prepared via carboxylic acid adsorption and photolysis. Phys Chem Chem Phys 14:3066–3074. https://doi.org/10.1039/C1CP22515D
Zhang Z, Cao K, Yates JT (2013) Defect-electron spreading on the TiO2(110) semiconductor surface by water adsorption. J Phys Chem Lett 4:674–679. https://doi.org/10.1021/jz400101f
Funding
This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science Program, FWP 47319. PNNL is a multiprogram national laboratory operated for DOE by Battelle under Contract DE-AC05-76RL01830. Computational resources were provided by a user proposal at the National Energy Research Scientific Computing Center located at Lawrence Berkley National Laboratory.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Connor, C.R., Ma, R., Collinge, G. et al. Insights into Acetic Acid Binding and Ketene Formation on Anatase TiO2(101). Top Catal 66, 1087–1101 (2023). https://doi.org/10.1007/s11244-023-01828-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01828-1