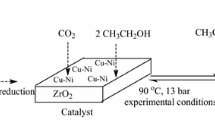
Abstract
The present research investigates the possibility of enhancing the stability of SO42−–ZrO2 catalyst by adding Cu (2–12 wt%) prepared via a simple one-pot synthesis method. The substantial changes in the structures of Cu are observed which can be classified into two different ranges of Cu loading: 2–8 wt% and 10–12 wt%. Cu2O is predominant phase at Cu loading of 2–8 wt%, which strongly interacts with ZrO2, resulting in a decrease of number of acid sites and thus lowering DME yield compared to bare SO42−–ZrO2. At Cu loading of 10–12 wt%, the predominant phase of Cu becomes CuSO4 and the SO42− from CuSO4 may be formed as bridging S–O–S bonds with another S–O bonded to Zr atom, resulting in the catalytic stability improvement. The optimum Cu loading is 10 wt% which attains the maximum DME yield of 3.2% at 260 °C and 20 bar.








Similar content being viewed by others
Data Availability
Authors can confirm that all relevant data are included in the article and/or its supplementary information files.
References
IEA (International Energy Agency) (2021) Net Zero by 2050: A roadmap for the global energy sector. https://www.iea.org/reports/net-zero-by-2050
Li Z, Men Y, Liu S, Wang J, Qin K, Tian D, Shi T, Zhang L, An W (2022) Boosting CO2 hydrogenation efficiency for methanol synthesis over Pd/In2O3/ZrO2 catalysts by crystalline phase effect. Appl Surf Sci 603:154420. https://doi.org/10.1016/j.apsusc.2022.154420
Chen G, Yu J, Li G, Zheng X, Mao H, Mao D (2023) Cu+–ZrO2 interfacial sites with highly dispersed copper nanoparticles derived from Cu@UiO-67 hybrid for efficient CO2 hydrogenation to methanol. Int J Hydrogen Energy 48:2605–2616. https://doi.org/10.1016/j.ijhydene.2022.10.172
Wang G, Mao D, Guo X, Yu J (2019) Methanol synthesis from CO2 hydrogenation over CuO–ZnO–ZrO2–MxOy catalysts (M=Cr, Mo and W). Int J Hydrogen Energy 44:4197–4207. https://doi.org/10.1016/j.ijhydene.2018.12.131
Hepburn C, Adlen E, Beddington J, Carter EA, Fuss S, Dowell NM, Minx JC, Smith P, Williams CK (2019) The technological and economic prospects for CO2 utilization and removal. Nature 575:87–97. https://doi.org/10.1038/s41586-019-1681-6
Kashyap D, Teller H, Subramanian P, Bĕlský P, Gebru MG, Pitussi I, Yadav RS, Kornweitz H, Schechter A (2022) Sn-based atokite alloy nanocatalyst for high-power dimethyl ether fueled low-temperature polymer electrolyte fuel cell. J Power Sources 544:231882. https://doi.org/10.1016/j.jpowsour.2022.231882
Golunski S, Burch R (2021) CO2 hydrogenation to methanol over copper catalysts: learning from syngas conversion. Top Catal 64:974–983. https://doi.org/10.1007/s11244-021-01427-y
Liang Y, Mao D, Guo X, Yu J, Wu G, Ma Z (2021) Solvothermal preparation of CuO–ZnO–ZrO2 catalysts for methanol synthesis via CO2 hydrogenation. J Taiwan Inst Chem Eng 121:81–91. https://doi.org/10.1016/j.jtice.2021.03.049
Phongamwong T, Chantaprasertporn U, Witoon T, Numpilai T, Poo-arporn Y, Limphirat W, Donphai W, Dittanet P, Chareonpanich M, Limtrakul J (2017) CO2 hydrogenation to methanol over CuO–ZnO–ZrO2–SiO2 catalysts: effects of SiO2 contents. Chem Eng J 316:692–703. https://doi.org/10.1016/j.cej.2017.02.010
Witoon T, Kachaban N, Donphai W, Kidkhunthod P, Faungnawakij K, Chareonpanich M, Limtrakul J (2016) Tuning of catalytic CO2 hydrogenation by changing composition of CuO–ZnO–ZrO2 catalysts. Energy Convers Manag 118:21–31. https://doi.org/10.1016/j.enconman.2016.03.075
Witoon T, Numpilai T, Phongamwong T, Donphai W, Boonyuen C, Warakulwit C, Chareonpanich M, Limtrakul J (2018) Enhanced activity, selectivity and stability of a CuO–ZnO–ZrO2 catalyst by adding graphene oxide for CO2 hydrogenation to methanol. Chem Eng J 334:1781–1791. https://doi.org/10.1016/j.cej.2017.11.117
Akkharaphatthawon N, Chanlek N, Cheng CK, Chareonpanich M, Limtrakul J, Witoon T (2019) Tuning adsorption properties of GaxIn2-xO3 catalysts for enhancement of methanol synthesis activity from CO2 hydrogenation at high reaction temperature. Appl Surf Sci 489:278–286. https://doi.org/10.1016/j.apsusc.2019.05.363
Cao A, Wang Z, Li H, Nørskov JK (2021) Relations between surface oxygen vacancies and activity of methanol formation from CO2 hydrogenation over In2O3 surfaces. ACS Catal 11:1780–1786. https://doi.org/10.1021/acscatal.0c05046
Numpilai T, Kidkhunthod P, Cheng CK, Wattanakit C, Chareonpanich M, Limtrakul J, Witoon T (2021) CO2 hydrogenation to methanol at high reaction temperatures over In2O3/ZrO2 catalysts: Influence of calcination temperatures of ZrO2 support. Catal Today 375:298–306. https://doi.org/10.1016/j.cattod.2020.03.011
Chafik T, Bianchi D, Teichner SJ (1995) On the mechanism of the methanol synthesis involving a catalyst based on zirconia support. Top Catal 2:103–116. https://doi.org/10.1007/BF01491959
Tada S, Ochiai N, Kinoshita H, Yoshida M, Shimada N, Joutsuka T, Nishijima M, Honma T, Yamauchi N, Kobayashi Y, Iyoki K (2022) Active sites on ZnxZr1-xO2-x solid solution catalysts for CO2-to-methanol hydrogenation. ACS Catal 12:7748–7759. https://doi.org/10.1021/acscatal.2c01996
Tada S, Li D, Okazaki M, Kinoshita H, Nishijima M, Yamauchi N, Kobayashi Y, Iyoki K (2022) Influence of Si/Al ratio of MOR type zeolites for bifunctional catalysts specific to the one-pass synthesis of lower olefins via CO2 hydrogenation. Catal Today. https://doi.org/10.1016/j.cattod.2022.06.043
Temvuttirojn C, Poo-arporn Y, Chanlek N, Cheng CK, Chong CC, Limtrakul J, Witoon T (2020) Role of calcination temperatures of ZrO2 support on methanol synthesis from CO2 hydrogenation at high reaction temperatures over ZnOx/ZrO2 catalysts. Ind Eng Chem Res 59:5525–5535. https://doi.org/10.1021/acs.iecr.9b05691
Osman AI, Abu-Dahrieh JK (2018) Kinetic Investigation of η-Al2O3 catalyst for dimethyl ether production. Catal Lett 148:1236–1245. https://doi.org/10.1007/s10562-018-2319-2
Sahebdelfar S, Bijani PM, Yaripour F (2022) Deactivation kinetics of γ-Al2O3 catalyst in methanol dehydration to dimethyl ether. Fuel 310:122443. https://doi.org/10.1016/j.fuel.2021.122443
Bonora G, Todaro S, Frusteri L, Majchrzak-Kucęba I, Wawrzyńczak D, Pászti Z, Tálas E, Tompos A, Ferenc L, Solt H, Cannilla C, Frusteri F (2021) Inside the reaction mechanism of direct CO2 conversion to DME over zeolite-based hybrid catalysts. Appl Catal B Environ 294:120255. https://doi.org/10.1016/j.apcatb.2021.120255
Catizzone E, Aloise A, Giglio E, Ferrarelli G, Bianco M, Migliori M, Giordano G (2021) MFI vs. FER zeolite during methanol dehydration to dimethyl ether: the crystal size plays a key role. Catal Commun 149:106214. https://doi.org/10.1016/j.catcom.2020.106214
Ren S, Li S, Klinghoffer N, Yu M, Liang X (2019) Effects of mixing methods of bifunctional catalysts on catalyst stability of DME synthesis via CO2 hydrogenation. Carbon Resour Convers 2:85–94. https://doi.org/10.1016/j.crcon.2019.03.002
Rodriguez-Vega P, Ateka A, Kumakiri I, Vicente H, Ereňa J, Aguayo AT, Bilbao J (2021) Experimental implementation of a catalytic membrane reactor for the direct synthesis of DME from H2+CO/CO2. Chem Eng Sci 234:116396. https://doi.org/10.1016/j.ces.2020.116396
Tariq A, Esquius JR, Davies TE, Bowker M, Taylor SH, Hutchings GJ (2021) Combination of Cu/ZnO methanol synthesis catalysts and ZSM-5 zeolites to produce oxygenates from CO2 and H2. Top Catal 64:965–973. https://doi.org/10.1007/s11244-021-01447-8
Xu M, Lunsford JH, Goodman DW, Bhattacharyya A (1997) Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts. Appl Catal A: Gen 149:289–301. https://doi.org/10.1016/S0926-860X(96)00275-X
Wang S, Pu J, Wu J, Liu H, Xu H, Li X, Wang H (2020) SO42-/ZrO2 as solid acid for the esterification of palmitic acid with methanol: effects of the calcination time and recycle method. ACS Omega 5:30139–30147. https://doi.org/10.1021/acsomega.0c04586
Yang H, Zhou Y, Tong D, Yang M, Fang K, Zhou C, Yu W (2020) Catalytic conversion of cellulose to reducing sugars over clay-based solid acid catalyst supported nanosized SO42-–ZrO2. Appl Clay Sci 185:105376. https://doi.org/10.1016/j.clay.2019.105376
Zhang X, Zhu Z, Sun X, Yang J, Gao H, Huang Y, Luo X, Liang Z, Tontiwachwuthikul P (2019) Reducing energy penalty of CO2 capture using Fe promoted SO42-/ZrO2/MCM-41 catalyst. Environ Sci Technol 53:6094–6102. https://doi.org/10.1021/acs.est.9b01901
Temvuttirojn C, Chuasomboon N, Numpilai T, Faungnawakij K, Chareonpanich M, Limtrakul J, Witoon T (2019) Development of SO42–ZrO2 acid catalysts admixed with a CuO–ZnO–ZrO2 catalyst for CO2 hydrogenation to dimethyl ether. Fuel 241:695–703. https://doi.org/10.1016/j.fuel.2018.12.087
Witoon T, Permsirivanich T, Kanjanasoontorn N, Akkaraphatawon C, Seubsai A, Faungnawakij K, Warakulwit C, Chareonpanich M, Limtrakul J (2015) Direct synthesis of dimethyl ether from CO2 hydrogenation over Cu–ZnO–ZrO2/SO42–ZrO2 hybrid catalysts: effects of sulfur-to-zirconia ratios. Catal Sci Technol 5:2347–2357. https://doi.org/10.1039/C4CY01568A
Fan J, Ning P, Song Z, Liu X, Wang L, Wang J, Wang H, Long K, Zhang Q (2018) Mechanistic aspects of NH3-SCR reaction over CeO2/TiO2–ZrO2-SO42- catalyst: In situ DRIFTS investigation. Chem Eng J 334:855–863. https://doi.org/10.1016/j.cej.2017.10.011
Wang P, Zhang W, Zhang Q, Xu Z, Yang C, Li C (2018) Comparative study of n-butane isomerization over SO42-/Al2O3–ZrO2 and HZSM-5 zeolites at low reaction temperatures. Appl Catal A Gen 550:98–104. https://doi.org/10.1016/j.apcata.2017.11.006
Witoon T, Numpilai T, Dolsiririttigul N, Chanlek N, Poo-arporn Y, Cheng CK, Ayodele BV, Chareonpanich M, Limtrakul J (2022) Enhanced activity and stability of SO42-/ ZrO2 by addition of Cu combined with CuZnOZrO2 for direct synthesis of dimethyl ether from CO2 hydrogenation. Int J Hydrogen Energy 47:41374–41385. https://doi.org/10.1016/j.ijhydene.2022.03.150
Bing L, Wang G, Yi K, Tian A, Wang F, Wu C (2018) One-pot synthesis of Cu–SAPO-34 catalyst using waste mother liquid and its application in the selective catalytic reduction of NO with NH3. Catal Today 316:37–42. https://doi.org/10.1016/j.cattod.2018.02.025
Witoon T, Chalorngtham J, Dumrongbunditkul P, Chareonpanich M, Limtrakul J (2016) CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: effects of zirconia phases. Chem Eng J 293:327–336. https://doi.org/10.1016/j.cej.2016.02.069
Morterra C, Cerrato G (1994) Brønsted acidity of a superacid sulfate-doped ZrO2 system. J Phys Chem 98:12373–12381. https://doi.org/10.1021/j100098a036
Morterra C, Cerrato G, Meligrana G, Signoretto M, Pinna F, Strukul G (2001) Catalytic activity and some related spectral features of yttria-stabilised cubic sulfated zirconia. Catal Lett 73:113–119. https://doi.org/10.1023/A:1016642804827
Bensitel M, Saur O, Lavalley JC, Morrow BA (1988) An infrared study of sulfated zirconia. Mater Chem Phys 19:147–156. https://doi.org/10.1016/0254-0584(88)90007-7
Hino M, Kurashige M, Matsuhashi H, Arata K (2006) The surface structure of sulfated zirconia: studies of XPS and thermal analysis. Thermochim Acta 441:35–41. https://doi.org/10.1016/j.tca.2005.11.042
Numpilai T, Wattanakit C, Chareonpanic M, Limtrakul J, Witoon T (2019) Optimization of synthesis condition for CO2 hydrogenation to light olefins over In2O3 admixed with SAPO-34. Energy Convers Manag 180:511–523. https://doi.org/10.1016/j.enconman.2018.11.011
Zhao W, Zhang B, Wang G, Guo H (2014) Methane formation route in the conversion of methanol to hydrocarbons. J Energy Chem 23:201–206. https://doi.org/10.1016/S2095-4956(14)60136-4
Kurmaev EZ, Fedorenko VV, Galakhov VR, Bartkowski S, Uhlenbrock S, Neumann M, Slater PR, Greaves C, Miyazaki Y (1996) Analysis of oxyanion (BO33-, CO32-, SO42-, PO43-, SeO44-) substitution in Y123 compounds studied by X-ray photoelectron spectroscopy. J Supercond 9:97–100. https://doi.org/10.1007/BF00728433
Acknowledgements
This research was supported in part by the Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand, project FF(KU)21.65 and the National Research Council of Thailand (N41A640081).
Author information
Authors and Affiliations
Contributions
TN: Investigation, Validation, Writing-Original draft preparation, Writing-Reviewing and Editing. ND: Investigation, Writing-Reviewing and Editing. AL: Visualization, Writing-Reviewing and Editing. CKC: Visualization, Writing-Reviewing and Editing. NC: Investigation and Validation for XPS analysis. YYP: Investigation and Validation for XANES analysis. TW: Investigation, Conceptualization, Methodology, Writing-Original draft preparation, Writing-Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Numpilai, T., Dolsiririttigul, N., Laobuthee, A. et al. One-Pot Synthesis of Cu–SO42−–ZrO2 Catalysts for Use as Acid Catalyst in Dimethyl Ether Production from CO2 Hydrogenation. Top Catal 66, 1467–1477 (2023). https://doi.org/10.1007/s11244-023-01814-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01814-7