Abstract
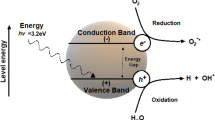
2,4-Dichlorophenol (2,4-DCP) is a persistent organic compound present in many water reservoirs for human consumption, generating a high risk for all species, especially for humans due to its toxicity. In this work, Mg/Al = 2, 3 and 4 layered double hydroxides activated at 450 °C are presented for the formation of photocatalysts formed by the mixture of MgO–MgAl2O4 oxides for the photocatalytic degradation of 2,4-DCP in aqueous solution in a UV irradiated reaction system. The results showed a good photocatalytic activity by achieving a complete degradation of 2,4-DCP up to mineralization. The high degradation capacity of 2,4-DCP is attributed to the presence of holes in these materials that promote the formation of hydroxyl radicals (OH⋅) and superoxide radicals (O2⋅−) in only 30 min of reaction time.

















Similar content being viewed by others
References
Gersich F, Milazzo D (1988) Chronic toxicity of aniline and 2,4-Dichlorophenol to daphnia magna straus. Bull Environ Contamin Toxicol 40:1–7
Kintz P, Tracqui A, Mangin P (1992) Accidental death caused by the absorption of 2,4-dichlorophenol through the skin. Arch Toxicol 66:298–299
Huang Z, Chen G, Zeng G, Guo Z, He K, Hu L, Wu J, Zhang L, Zhu Y, Song Z (2017) Toxicity mechanisms and synergies of silver nanoparticles in 2,4-dichlorophenol degradation by Phanerochaete chrysosporium. J Hazard Mater 321:37–46. https://doi.org/10.1016/j.jhazmat.2016.08.075
Yuan C, Zhang C, Qi Y, Li D, Hu Y, Huang D (2020) 2,4-Dichlorophenol induced feminization of zebrafish by down-regulating male-related genes through DNA methylation. Ecotoxicol Environ Saf 189:110042. https://doi.org/10.1016/j.ecoenv.2019.110042
Chapman K, Wilde E, Chapman F, Verma J, Shah U, Stannard L, Seager A, Tonkin J, Brown M, Doherty A, Johnson G, Doak S, Jenkins G (2021) Multiple-endpoint in vitro carcinogenicity test in human cell line TK6 distinguishes carcinogens from non-carcinogens and highlights mechanisms of action. Arch Toxicol 95:321–336. https://doi.org/10.1007/s00204-020-02902-3
Foszpańczyk M, Drozdek E, Gmurek M, Ledakowicz S (2018) Toxicity of aqueous mixture of phenol and chlorophenols upon photosensitized oxidation initiated by sunlight or vis-lamp. Environ Sci Pollut Res 25:34968–34975. https://doi.org/10.1007/s11356-018-1286-x
Soomro F, Memon S, Memon N (2020) Khuhawar M (2020) A new Schiff’s base polymer for remediation of phenol, 2-chlorophenol and 2,4-dichlorophenol from contaminated aqueous systems. Polym Bull 77:2367–2383. https://doi.org/10.1007/s00289-019-02852-6
Kuśmierek K, Świątkowski A, Skrzypczyńska K, Błażewicz S, Hryniewicz S (2017) The effects of the thermal treatment of activated carbon on the phenols adsorption. Korean J Chem Eng 34:1081–190. https://doi.org/10.1007/s11814-017-0015-3
Das Neves L, Ohara-Miyamura T, Moraes D, Vessoni-Penna T, Converti A (2006) Biofiltration methods for the removal of phenolic residues. Appl Biochem Biotechnol 129–132:130–152. https://doi.org/10.1385/abab:129:1:130
Oluwole A, Omotola E, Olatunji O (2020) Pharmaceuticals and personal care products in water and wastewater: a review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem 14(62):1–19. https://doi.org/10.1186/s13065-020-00714-1
Karci A, Arslan-Alaton I, Olmez-Hanci T, Bekbölet M (2012) Transformation of 2,4-dichlorophenol by H2O2/UV-C, Fenton and photo-Fenton processes: oxidation products and toxicity. J Photochem Photobiol A 230:65–73. https://doi.org/10.1016/j.jphotochem.2012.01.003
Aken P, Broeck R, Degrève J (2015) The effect of ozonation on the toxicity and biodegradability of 2,4-dichlorophenol-containing wastewater. Chem Eng J 280:728–736. https://doi.org/10.1016/j.cej.2015.06.019
Li X, Zhao W, Zhao J (2002) Visible light-sensitized semiconductor photocatalytic degradation of 2,4-dichlorophenol. Sci China Ser B 45:421–425
Yan N, An M, Chu J, Cao L, Zhu G, Wu W, Wang L, Zhang Y, Rittmann B (2021) More rapid dechlorination of 2,4-dichlorophenol using acclimated bacteria. Bioresour Technol 326:124738. https://doi.org/10.1016/j.biortech.2021.124738
Zhang Z, Hu Y, Ruan W, Ai H, Yuan B, Fu M (2021) Highly improved dechlorination of 2,4-dichlorophenol in aqueous solution by Fe/Ni nanoparticles supported by polystyrene resin. Chemosphere 266:128976. https://doi.org/10.1016/j.chemosphere.2020.128976
Ruan X, Liu H, Wang J, Zhao D, Fan X (2019) A new insight into the main mechanism of 2,4-dichlorophenol dechlorination by Fe/Ni nanoparticles. Sci Total Environ 697:133996. https://doi.org/10.1016/j.scitotenv.2019.133996
Tamaki T, Nakanishi N, Ohashi H, Yamaguchi T (2012) The effect of particle size and surface area on the ion conductivity of layered double hydroxide. Electrochem Commun 25:50–53. https://doi.org/10.1016/j.elecom.2012.09.003
Wang Q, O’Hare D (2012) Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev 112:4124–4155. https://doi.org/10.1021/cr200434v
Mohapatra L, Parida K (2016) A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J Mater Chem A 4:10744–10766. https://doi.org/10.1039/C6TA01668E
Takehira K (2017) Recent development of layered double hydroxide-derived catalysts—rehydration, reconstitution, and supporting, aiming at commercial application. Appl Clay Sci 136:112–141. https://doi.org/10.1016/j.clay.2016.11.012
Taoufik N, Sadiq M, Abdennouri M, Qourzal S, Khaaee A, Sillanpää M, Barka N (2022) Materials recent advances in the synthesis and environmental catalytic applications of layered double hydroxides-based materials for degradation of emerging pollutants through advanced oxidation processes. Res Bull 154:111924. https://doi.org/10.1016/j.materresbull.2022.111924
Védrine J (2019) Recent developments and prospectives of acid-base and redox catalytic processes by metal oxides. Appl Catal A 575:170–179. https://doi.org/10.1016/j.apcata.2019.02.012
Kim S, Durand P, André E, Carteret C (2017) Enhanced photocatalytic ability of Cu, Co doped ZnAl based mixed metal oxides derived from layered double hydroxides. Colloids Surf A 524:43–52. https://doi.org/10.1016/j.colsurfa.2017.04.019
Pera-Titus M, Garcı́a-Molina V, Baños M, Giménez J, Esplugas S (2004) Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl Catal B 47:219–256. https://doi.org/10.1016/j.apcatb.2003.09.010
Chowdhury P, Bhattacharyya K (2016) Synthesis and characterization of Mn/Co/Ti LDH and its utilization as a photocatalyst in visible light assisted degradation of aqueous Rhodamine B. RSC Adv 6:112016–112034. https://doi.org/10.1039/c6ra24288j
Olfs H, Torres-Dorante L, Eckelt E, Kosslick H (2009) Comparison of different synthesis routes for Mg–Al layered double hydroxides (LDH): characterization of the structural phases and anion exchange properties. Appl Clay Sci 43:459–464. https://doi.org/10.1016/j.clay.2008.10.009
Mohd R, Zuchra H, Martunus W, Weeramundage J (2009) Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: a review. Appl Organomet Chem 23(9):335–346. https://doi.org/10.1002/aoc.1517
Otgonjargal E, Burmaa G, Enkhma B, Enkhtuul M, Nyam-Ochir L, Ryu S, Khasbaatar D (2014) Study on synthesizing Mg/Al layered double hydroxides at different pHs. Mongolian J Chem 15(41):36–39. https://doi.org/10.5564/mjc.v15i0.319
Klemkaite K, Prosycevas I, Taraskevicius R, Khinsky A, Kareiva A (2011) Synthesis and characterization of layered double hydroxides with different cations (Mg Co, Ni, Al), decomposition and reformation of mixed metal oxides to layered structures. Cent Eur J Chem 9(2):275–28. https://doi.org/10.2478/s11532-011-0007-9
Wang Q, Yan Q, Zhao Y, Ren J, Ai N (2022) Preparation of amine-modified Cu-Mg-Al LDH composite photocatalyst. Nanomaterials 12(127):1–17. https://doi.org/10.3390/nano120101
Silva L, Anchieta C, Duarte J, Meili L, Freire J (2021) Effect of drying on the fabrication of MgAl layered double hydroxides. ACS Omega 6:21819–21829. https://doi.org/10.1021/acsomega.1c03581
Ma S, Fan C, Huang G, Li Y, Yang X, Ooi K (2010) Origin of CO32—shortage in MgAl layered double hydroxides with Mg/Al < 2. Eur J Inorg Chem 14:2079–2083. https://doi.org/10.1002/ejic.201000010
An S, Kwon D, Cho J, Jung J (2020) Effect of the solvent on the basic properties of Mg–Al hydrotalcite catalysts for glucose isomerization. Catalysts. https://doi.org/10.3390/catal10111236
Matusik J, Hyla J, Maziarz P, Rybka K, Leiviskä T (2019) Performance of halloysite-Mg/Al LDH materials for aqueous As(V) and Cr(VI) removal. Materials 12:3569. https://doi.org/10.3390/ma12213569
Mahmoud R, Taha M, Zaher A, Amin R (2021) Understanding the physicochemical properties of Zn–Fe LDH nanostructure as sorbent material for removing of anionic and cationic dyes mixture. Sci Rep 11:21365. https://doi.org/10.1038/s41598-021-00437-w
Kumar A, Pandey G (2017) A review on the factors affecting the photocatalytic degradation of hazardous materials. Mater Sci Eng Int J 1(3):106–114. https://doi.org/10.15406/mseij.2017.01.00018
Montenegro-Ayo R, Morales-Gomero J, Alarcon H, Corzo A, Westerhoff P, Garcia-Segura S (2021) Photoelectrocatalytic degradation of 2,4-dichlorophenol in a TiO2 nanotube-coated disc flow reactor. Chemosphere 268:129320. https://doi.org/10.1016/j.chemosphere.2020.129320
Tzompantzi F, Castillo-Rodríguez JC, Tzompantzi-Flores C, Pérez-Hernández R, Gómez R, Santolalla-Vargas CE, Ramos-Ramírez E (2022) Addition of SnO2 over an oxygen deficient zirconium oxide (ZrxOy) and its catalytic evaluation for the photodegradation of phenol in water. Catal Today 394:376–389. https://doi.org/10.1016/j.cattod.2021.07.027
Tzompantzi-Flores C, Castillo-Rodríguez JC, Gómez R, Tzompantzi F, Pérez-Hernández R, De la Luz Tlapaya V, Santolalla-Vargas CE (2019) Synthesis and characterization of ZnZr composites for the photocatalytic degradation of phenolic molecules: addition effect of ZrO2 over hydrozincite Zn5(OH)6(CO3)2. J Chem Technol Biotechnol 94(11):3428–3439. https://doi.org/10.1002/jctb.5928
Acknowledgements
We would like to especially thank the Directorate for Research and Postgraduate Support (DAIP) at the University of Guanajuato for their support in developing this project. Additionally, we thank the CONACyT for the graduate scholarship and to UAM-I for technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramos-Ramírez, E., Gutiérrez-Ortega, N., Tzompantzi-Morales, F. et al. Photocatalytic Degradation of 2,4-Dichlorophenol in Water Using MgAl Activated Hydrotalcites as Photocatalyst. Top Catal 65, 1469–1481 (2022). https://doi.org/10.1007/s11244-022-01688-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01688-1