Abstract
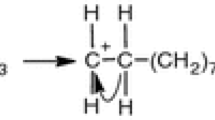
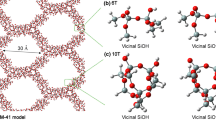
The most likely routes for the thermal decomposition of biomass derived D-galacturonic acid leading to furfural were analyzed through comparison of the activation energy barriers associated with possible kinetic pathways. Dehydration steps were associated with significant activation barriers. The effect of a mesoporous catalyst with Brønsted acidity such as Al-SBA-15 was analyzed. The chemical structure of SBA-15 was optimized by means of a comparison from the experimental structural data previously reported in literature and the structural parameters obtained from DFT calculations at B3LYP/6–31 + G (d, p) level of several catalyst surface models. The bicyclic model structure obtained proved to be representative of framework species in SBA-15. The isomorphic substitution of Al in the SBA-15 model structure was made in order to generate Brønsted acidity. The acidity was studied for the dehydration of methanol, this reaction being representative of the dehydration processes that occur in biomass transformation to furfural. A higher Brønsted acidity strength was observed in free silanol type (Q3) and geminal silanol type (Q2) sites, formed by the isomorphic Al substitution of Si.













Similar content being viewed by others
References
Hanif MU, Capareda SC, Kongkasawan J, Iqbal H, Arazo RO, Baig MA (2016) Correction: effects of pyrolysis temperature on product yields and energy recovery from co-feeding of cotton gin trash, cow manure, and microalgae: a simulation study. PLoS ONE 11(7):1–11. https://doi.org/10.1371/journal.pone.0156565
Mariscal R, Maireles-Torres P, Ojeda M, Sádaba I, López Granados M (2016) Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9(4):1144–1189. https://doi.org/10.1039/C5EE02666K
Pfaltzgraff LA, De Bruyn M, Cooper EC, Budarin V, Clark JH (2013) Food waste biomass: a resource for high-value chemicals. Green Chem 15(2):307–314. https://doi.org/10.1039/c2gc36978h
Edwards MC, Doran-Peterson J (2012) Pectin-rich biomass as feedstock for fuel ethanol production. Appl Microbiol Biotechnol 95(3):565–575. https://doi.org/10.1007/s00253-012-4173-2
Raveendran K, Ganesh A, Khilar KC (1996) Pyrolysis characteristics of biomass and biomass components. Fuel 75(8):987–998. https://doi.org/10.1016/0016-2361(96)00030-0
Manyà JJ, Velo E, Puigjaner L (2003) Kinetics of biomass pyrolysis: a reformulated three-parallel-reactions model. Ind Eng Chem Res 42(3):434–441. https://doi.org/10.1021/ie020218p
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86(12–13):1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Hosoya T, Kawamoto H, Saka S (2007) Cellulose-hemicellulose and cellulose-lignin interactions in wood pyrolysis at gasification temperature. J Anal Appl Pyrolysis 80(1):118–125. https://doi.org/10.1016/j.jaap.2007.01.006
Collard FX, Blin J (2014) A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev 38:594–608. https://doi.org/10.1016/j.rser.2014.06.013
Torres-García E, Ramírez-Verduzco LF, Aburto J (2020) Pyrolytic degradation of peanut shell: activation energy dependence on the conversion. Waste Manage 106:203–212. https://doi.org/10.1016/j.wasman.2020.03.021
Aburto J, Moran M, Galano A, Torres-García E (2015) Non-isothermal pyrolysis of pectin: a thermochemical and kinetic approach. J Anal Appl Pyrolysis 112:94–104. https://doi.org/10.1016/j.jaap.2015.02.012
Aho A, Salmi T, Murzin DY (2013) Catalytic pyrolysis of lignocellulosic biomass. Role Catal Sustain Prod Bio-Fuels Bio-Chem. https://doi.org/10.1016/B978-0-444-56330-9.00005-X
Kan T, Strezov V, Evans TJ (2016) Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sustain Energy Rev 57:126–1140. https://doi.org/10.1016/j.rser.2015.12.185
Thangalazhy-Gopakumar S, Adhikari S, Gupta RB (2012) Catalytic pyrolysis of biomass over H +ZSM-5 under hydrogen pressure. Energy Fuels 26(8):5300–5306. https://doi.org/10.1021/ef3008213
Anderson JR, Mole T, Christov V (1980) Mechanism of some conversions over ZSM-5 catalyst. J Catal 61(2):477–484. https://doi.org/10.1016/0021-9517(80)90394-2
Lee K, Gu GH, Mullen CA, Boateng AA, Vlachos DG (2015) Guaiacol hydrodeoxygenation mechanism on Pt(111): Insights from density functional theory and linear free energy relations. Chemsuschem 8(2):315–322. https://doi.org/10.1002/cssc.201402940
Hu W et al (2006) Acid sites in mesoporous Al-SBA-15 material as revealed by solid-state NMR spectroscopy. Microporous Mesoporous Mater 92(1–3):22–30. https://doi.org/10.1016/j.micromeso.2005.12.013
Koekkoek A, van Veen J, Gerritsen P, Giltay P, Magusin P, Hensen E (2012) Brønsted acidity of Al/SBA-15. Microporous Mesoporous Mater 151:34–43. https://doi.org/10.1016/j.micromeso.2011.11.019
Galano A, Rodriguez-Gattorno G, Torres-García E (2008) A combined theoretical-experimental study on the acidity of WO x-ZrO2 systems. Phys Chem Chem Phys 10(28):4181–4188. https://doi.org/10.1039/b802934b
IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) (1999) Nomenclature of glycolipids: recommendations 1997. Glycoconj J 16(1):1–6. https://doi.org/10.1023/A:1017225000910
Scheraga H, Sundaralingam M, Sundararajan P (1983) Symbols for specifying the conformation of polysaccharide chains (Recommendations 1981). Pure Appl Chem 55(8):1269–1272. https://doi.org/10.1351/pac198355081269
Gallo JMR, Alonso DM, Mellmer MA, Yeap JH, Wong HC, Dumesic JA (2013) Production of furfural from lignocellulosic biomass using beta zeolite and biomass-derived solvent. Top Catal 56(18–20):1775–1781. https://doi.org/10.1007/s11244-013-0113-3
Danon B, Marcotullio G, de Jong W (2014) Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem 16(1):39–54. https://doi.org/10.1039/C3GC41351A
Perlin A (2011) Thermal decarboxylation of uronic acids. Can J Chem 30:278–290. https://doi.org/10.1139/v52-039
Kruk M, Jaroniec M, Ko CH, Ryoo R (2000) Characterization of the porous structure of SBA-15. Chem Mater 12(7):1961–1968. https://doi.org/10.1021/cm000164e
Sonwane CG, Li Q (2005) Molecular simulation of RMM: ordered mesoporous SBA-15 type material having microporous ZSM-5 walls. J Phys Chem B 109(38):17993–17997. https://doi.org/10.1021/jp053360n
Susman S et al (1991) Intermediate-range order in permanently densified vitreous SiO2: a neutron-diffraction and molecular-dynamics study. Phys Rev B 43(1):1194–1197. https://doi.org/10.1103/PhysRevB.43.1194
Malfait WJ, Halter WE, Verel R (2008) 29Si NMR spectroscopy of silica glass: T1 relaxation and constraints on the Si-O-Si bond angle distribution. Chem Geol 256(3–4):269–277. https://doi.org/10.1016/j.chemgeo.2008.06.048
Dongyuan Zhao QH, Feng J et al (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 Angstrom pores. Science 279(5350):548–552. https://doi.org/10.1126/science.279.5350.548
Li Y et al (2007) Direct synthesis of Al−SBA-15 mesoporous materials via hydrolysis-controlled approach. J Phys Chem B 108(28):9739–9744. https://doi.org/10.1021/jp049824j
Wang Z, Wang D, Zhao Z, Chen Y, Lan J (2011) A DFT study of the structural units in SBA-15 mesoporous molecular sieve. Comput Theor Chem 963(2–3):403–411. https://doi.org/10.1016/j.comptc.2010.11.004
Wu W, Cai J, Liu R (2013) Isoconversional kinetic analysis of distributed activation energy model processes for pyrolysis of solid fuels. Ind Eng Chem Res 52(40):14376–14383. https://doi.org/10.1021/ie4021123
Acknowledgements
The authors gratefully acknowledge the Mexican Council for Science and Technology, CONACYT and the support of the lab technician, Jorge Alfredo Rangel Jimenez. This work was supported by the project: “Obtención de biocombustibles y compuestos químicos de alto valor agregado a partir de biomasas de desecho ricas en pectina”, CONACYT agreement: CB 255527-2016, Also, the Fellowship with reference number: 586594 from the Mexican Council for Science and Technology, CONACYT was assigned to Paulina Rocha Sanchez as PhD student.
Funding
This work was supported by the project: “Obtención de biocombustibles y compuestos químicos de alto valor agregado a partir de biomasas de desecho ricas en pectina”, CONACYT agreement: CB 255527-2016; the Fellowship with reference number: 586594 from the Mexican Council for Science and Technology, CONACYT was assigned to Paulina Rocha Sanchez as PhD student.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Simulation, data collection and analysis were performed by Paulina Rocha Sánchez. The first draft of the manuscript was written by Paulina Rocha Sánchez and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rocha-Sánchez, P., Cárdenas-Galindo, MG. Theoretical Study on the Brønsted Acidity of Al Doped SBA-15 for Methanol Dehydration as a Reaction Analog for the Transformation of Pectin to Furfural. Top Catal 65, 1482–1494 (2022). https://doi.org/10.1007/s11244-022-01669-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01669-4