Abstract
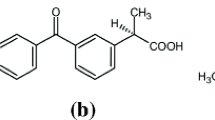
Lipases are enzymes widely applied in the kinetic resolution of racemic non-steroidal anti-inflammatory drugs (NSAIDs). The esterification of racemic ibuprofen and ketoprofen with glycerol catalyzed by the lipase B of Candida antarctica (CALB) was performed by some of us, achieving very different results for both NSAIDs in terms of enzymatic activity, enantio- and regioselectivity. A molecular modelling investigation allowed to establish the steric energies and the enthalpy variations along the diffusion of glycerol through the enzymatic tunnel towards the active catalytic triad of the lipase along with the interaction with the acyl enzyme species. In this context, it was possible to demonstrate that glycerol approaching the acyl enzyme of the R-ibuprofen possesses lower steric hindrance than the S-ibuprofen acyl enzyme (− 185.3 vs − 188.6 kcal mol−1 for S and R-enantiomers, in average). Although, the steric energy is somehow similar when the acyl enzyme of the R/S-ketoprofen is considered (− 201 kcal mol−1) the proximity of glycerol to the aminoacid residues of the enzymatic tunnel towards the active site plays a key role. In fact, the closer distance of glycerol to the tunnel walls when the acyl enzyme of ketoprofen is present than ibuprofen (2.1 Å vs 2.9 Å) allows multiple H-bonding interactions between the polyol and the aminoacids and also increases the enthalpy of formation of glycerol–acyl enzyme species.









Similar content being viewed by others
References
Illanes A (2008) Enzyme biocatalysis: principles and applications. Springer, Berlin
Gonçalves Filho D, Gonçalves Silva A, Zanella Guidini C (2019) Lipases: sources, immobilization methods, and industrial applications. Appl Microbiol Biotechnol 103:7399–7423. https://doi.org/10.1007/s00253-019-10027-6
Uppenberg J, Hansen MT, Patkar S, Jones TA (1994) The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 2:293–308. https://doi.org/10.1016/S0969-2126(00)00031-9
Gora A, Brezovsky J, Damborsky J (2013) Gates of enzymes. Chem Rev 113:5871–5923. https://doi.org/10.1021/cr300384w
Lu C, Peng X, Lu D, Liu Z (2020) Global and kinetic profiles of substrate diffusion in Candida antarctica lipase B: molecular dynamics with the Markov-state model. ACS Omega 5:9806–9812. https://doi.org/10.1021/acsomega.9b04432
Foresti ML, Galle M, Ferreira ML, Briand LE (2009) Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: experimental and molecular modeling aspects. J Chem Technol Biotechnol 84:1461–1473. https://doi.org/10.1002/jctb.2200
Toledo MV, José C, Collins SE, Bonetto RD, Ferreira ML, Briand LE (2012) Esterification of R/S-ketoprofen with 2-propanol as reactant and solvent catalyzed by Novozym® 435 at selected conditions. J Mol Catal B 83:108–119. https://doi.org/10.1016/j.molcatb.2012.06.016
Toledo MV, José C, Collins SE, Ferreira ML, Briand LE (2015) Towards a green enantiomeric esterification of R/S-ketoprofen: a theoretical and experimental investigation. J Mol Catal B 118:52–61. https://doi.org/10.1016/j.molcatb.2015.05.003
Toledo MV, José C, Llerena Suster CR, Collins SE, Portela R, Bañares MA, Briand LE (2021) Catalytic and molecular insights of the esterification of ibuprofen and ketoprofen with glycerol. Mol Catal 513:111811. https://doi.org/10.1016/j.mcat.2021.111811
Braham SA, Siar EH, Arana-Peña S, Bavandi H, Carballares D, Morellon-Sterling R, de Andrades D, Jkornecki JK, Fernandez-Lafuente R (2021) Positive effect of glycerol on the stability of immobilized enzymes: is it a universal fact? Process Biochem 102:108–121. https://doi.org/10.1016/j.procbio.2020.12.015
Kim MG, Lee EG, Chung BH (2000) Improved enantioselectivity of Candida rugosa lipase towards ketoprofen ethyl ester by a simple two-step treatment. Process Biochem 35:977–982. https://doi.org/10.1016/S0032-9592(00)00129-1
De Olivera EB, Humeau C, Chebil L, Maia ER, Dehez F, Maigret B, Ghoul M, Engasser J-M (2009) A molecular modelling study to rationalize the regioselectivity in acylation of flavonoid glycosides catalyzed by Candida antarctica lipase B. J Mol Catal B 59:96–105. https://doi.org/10.1016/j.molcatb.2009.01.011
José C, Toledo MV, Osorio Grisales J, Briand LE (2014) Effect of co-solvents in the enantioselective esterification of (R/S)-ibuprofen with ethanol. Curr Catal 3:131–138
Gudiño ED, Iglesias LE, Ferreira ML (2012) A rational approach to the regioselective deacetylation of 2′,3′,5′-tri-O-acetyluridineby Novozym 435 catalysed alcoholysis. Biochim Biophys Acta 1824:627–636. https://doi.org/10.1016/j.bbapap.2012.01.009
Stewart JJP (1989) Optimization of parameters for semiempirical methods I. Method J Comput Chem 10:209–220. https://doi.org/10.1002/jcc.540100208
Stewart JJP (1989) Optimization of parameters for semiempirical methods II. Appl J Comput Chem 10:221–264. https://doi.org/10.1002/jcc.540100209
Wu Y-Y, Zhao F-Q, Ju X-H (2014) A comparison of the accuracy of semi-empirical PM3, PDDG and PM6 methods in predicting heats of formation for organic compounds. J Mex Chem Soc 58:223–229
Galmés MA, Świderek K, Moliner V (2021) Computational studies suggest promiscuous Candida antarctica lipase B as an environmentally friendly alternative for the production of epoxides. J Chem Inf Model 61:3604–3614. https://doi.org/10.1021/acs.jcim.1c00425
Lonsdale R, Houghton KT, Żurek J, Bathelt CM, Foloppe N, de Groot MJ, Harvey JN, Mulholland AJ (2013) Quantum mechanics/molecular mechanics modeling of regioselectivity of drug metabolism in cytochrome P450 2C9. J Am Chem Soc 135:8001–8015. https://doi.org/10.1021/ja402016p
Elsässer B, Goettig P (2021) Mechanisms of proteolytic enzymes and their inhibition in QM/MM studies. Int J Mol Sci 22:3232. https://doi.org/10.3390/ijms22063232
Ainsley J, Lodol A, Mulholland AJ, Christo CZ, Karabencheva-Christova TG (2018) Chapter one—Combined quantum mechanics and molecular mechanics studies of enzymatic reaction mechanisms. Adv Protein Chem Struct Biol 113:1–32. https://doi.org/10.1016/bs.apcsb.2018.07.001
Lavandera I, Fernandez S, Magdalena J, Ferrero M, Kazlauskas RJ, Gotor V (2005) An inverse substrate orientation for the regioselective acylation of 3′,5′-diaminonucleosides catalyzed by Candida antarctica lipase B. ChemBioChem 6:1381–1390. https://doi.org/10.1002/cbic.200400422
Ortega-Rojas M, Castillo E, Said Razo-Hernández R, Pastor N, Juaristi E, Escalante J (2021) Effect of the substituent and amino group position on the lipase-catalyzed resolution of γ-amino esters: a molecular docking study shedding light on Candida antarctica lipase B enantioselectivity. Eur J Org Chem 2021:4790–4802. https://doi.org/10.1002/ejoc.202100712
Toledo MV, Llerena Suster CR, Ferreira ML, Collins SE, Briand LE (2017) Molecular recognition of an acyl–enzyme intermediate on the lipase B from Candida antarctica. Catal Sci Technol 7:1953–1964
Towey JJ, Soperb AK, Dougan L (2011) The structure of glycerol in the liquid state: a neutron diffraction study. Phys Chem Chem Phys 13:9397–9406. https://doi.org/10.1039/C0CP02136A
Sánchez DA, Tonetto GM, Ferreira ML (2016) An insight on acyl migration in solvent-free ethanolysis of model triglycerides using Novozym 435. J Biotechnol 220(92):99
Vueba ML, Pina ME, Veiga F, Sousa JJ, Batista de Carvalho LAE (2006) Conformational study of ketoprofen by combined DFT calculations and Raman spectroscopy. Int J Pharm 307:56–65. https://doi.org/10.1016/j.ijpharm.2005.09.019
Vueba ML, Pina ME, BatistadeCarvalho LAE (2008) Conformational stability of ibuprofen: assessed by DFT calculations and optical vibrational spectroscopy. J Pharm Sci 97:845–859. https://doi.org/10.1002/jps.21007
Chem 3D Cambridge Soft CS ChemOffice Manual.
Acknowledgements
The authors acknowledge the financial support of CONICET and different grants (PIP No. 11220200102016CO), Universidad Nacional de La Plata (Project 11X-898) and ANPCyT (Grant Nos. PICT-2018-3762 and PICT 2018-03425).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toledo, M.V., Briand, L.E. & Ferreira, M.L. A Simple Molecular Model to Study the Substrate Diffusion into the Active Site of a Lipase-Catalyzed Esterification of Ibuprofen and Ketoprofen with Glycerol. Top Catal 65, 944–956 (2022). https://doi.org/10.1007/s11244-022-01636-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01636-z