Abstract
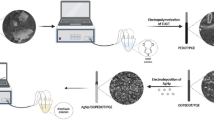
Ceftizoxime (CFX) is used to reduce the infection caused by both gram-negative and gram-positive bacteria. In this report, a novel electrochemical sensor for CFX comprising a Cu(Him)2 nanoparticles and ionic liquid (IL) hybride modified carbon paste electrode (CPE) has been developed. The structural properties of Cu(Him)2 nanoparticles was characterized using energy-dispersive X-ray spectroscopy (EDX) analyses, X-ray diffraction (XRD), and field emission scanning electron microscopy (FESEM). The results illustrate that Cu(Him)2/ILCPE exhibits an excellent electrocatalytic effect in the electrooxidation of CFX that leads to a considerable improvement in the corresponding anodic peak current. Under the best experimental conditions, the sensor exhibited a linear response to CFX from 2.0 to 1000.0 μM, with a limit of detection (LOD) of 0.5 nM. Finally, this also allows the development of a highly sensitive voltammetric sensor for the determination of CFX in pharmaceutical and biological samples.









Similar content being viewed by others
References
Azadmehr F, Zarei K (2020) Ultrasensitive determination of ceftizoxime using pencil graphite electrode modified by hollow gold nanoparticles/reduced graphene oxide. Arab J Chem 13:1890–1900
Beytur M, Kardaş F, Akyıldırım O, Özkan A, Bankoğlu B, Yüksek H, Atar N (2018) A highly selective and sensitive voltammetric sensor with molecularly imprinted polymer based silver@gold nanoparticles/ionic liquid modified glassy carbon electrode for determination of ceftizoxime. J Mol Liq 251:212–217
Wang L, Zheng X, Zhong W, Chen J, Jiang J, Hu P (2016) Validation and application of an LC–MS–MS method for the determination of ceftizoxime in human serum and urine. J Chromatogram Sci 54:713–719
Shahrokhian S, Ranjbar S, Ghalkhani M (2016) Modification of the electrode surface by Ag nanoparticles decorated nano diamond-graphite for voltammetric determination of ceftizoxime. Electroanalysis 28:469–476
Suzuki A, Noda K, Noguchi H (1980) High-performance liquid chromatographic determination of ceftizoxime, a new cephalosporin antibiotic, in rat serum, bile and urine. J Chromatogr B 182:448–453
Sanli S, Sanli N, Gumustas M, Ozkan SA, Karadas N, Aboul-Enein HY (2011) Simultaneous estimation of ceftazidime and ceftizoxime in pharmaceutical formulations by HPLC method. Chromatographia 74:549
Mccormick EM, Echols RM, Rosano TG (1984) Liquid chromatographic assay of ceftizoxime in sera of normal and uremic patients. Antimicrob Agents Chemother 25:336–338
Al-Momani IF (2001) Spectrophotometric determination of selected cephalosporins in drug formulations using flow injection analysis. J Pharma Biomed Anal 25:751–757
El Walily AFM, Gazy AAK, Belal SF, Khamis EF (2000) Use of cerium (IV) in the spectrophotometric and spectrofluorimetric determinations of penicillins and cephalosporins in their pharmaceutical preparations. Spectros Lett 33:931–948
Ojani R, Raoof JB, Zamani S (2010) A novel sensor for cephalosporins based on electrocatalytic oxidation by poly (o-anisidine)/SDS/Ni modified carbon paste electrode. Talanta 81:1522–1528
Owens GS, Lacourse WR (1996) Pulsed electrochemical detection of sulfur-containing compounds following microbore liquid chromatography. Curr Sep 14:82–89
Yola ML, Göde C, Atar N (2017) Molecular imprinting polymer with polyoxometalate/carbon nitride nanotubes for electrochemical recognition of bilirubin. Electrochim Acta 246:135–140
Kardaş F, Beytur M, Akyıldırım O, Yüksek H, Yola ML, Atar N (2017) Electrochemical detection of atrazine in wastewater samples by copper oxide (CuO) nanoparticles ionic liquid modified electrode. J Mol Liq 248:360–363
Srivastava AK, Upadhyay SS, Rawool CR, Punde NS, Rajpurohit AS (2019) Voltammetric techniques for the analysis of drugs using nanomaterials based chemically modified electrodes. Curr Anall Chem 15:249–276
Tajik S, Beitollahi H, Garkani Nejad F, Sheikhshoaie I, Sugih Nugraha A, Won Jang H, Yamauchi Y, Shokouhimehr M (2021) Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J Mater Chem A 9:8195–8220
Srikanta S, Parmeswara Naik P, Krishanamurthy G (2019) Electrochemical behaviour of 5-methoxy-5,6-bis(3-nitropheyl-4,5-dihydro-1,2,4-triazine-3(2H))-thione in presence of salicylaldehyde on zinc cathode with surface morphology and biological activity. Asian J Green Chem 4:149–158
Siddeeg SM, Alsaiari NS, Tahoon MA, Rebah FB (2020) The application of nanomaterials as electrode modifiers for the electrochemical detection of ascorbic acid. Int J Electrochem Sci 15:3327–3346
Elobeid WH, Elbashir AA (2019) Development of chemically modified pencil graphite electrode based on benzo-18-crown-6 and multi-walled CNTs for determination of lead in water samples. Prog Chem Biochem Res 2:24–33
Rshad S, Mofidi Rasi R (2019) Electrocatalytic oxidation of sulfite Ion at the surface carbon ceramic modified electrode with prussian blue. Eurasian Chem Commun 1:43–52
Nejad FG, Tajik S, Beitollahi H, Sheikhshoaie I (2021) Magnetic nanomaterials based electrochemical (bio) sensors for food analysis. Talanta 228:122075
Taei M, Salavati H, Fouladgar M, Abbaszadeha E (2020) Simultaneous determination of sunset yellow and tartrazine in soft drinks samples using nanocrystallites of spinel ferrite-modified electrode. Quarte J Iran Chem Commun 8:67–79
Rabiee N, Safarkhani M, Rabiee M (2018) Ultra-sensitive electrochemical on-line determination of Clarithromycin based on Poly (L-Aspartic acid)/graphite oxide/pristine graphene/glassy carbon electrode. Asian J Nanosci Mater 1:63–73
Tajik S, Beitollahi H, Asl MS, Jang HW, Shokouhimehr M (2021) BN-Fe3O4-Pd nanocomposite modified carbon paste electrode: Efficient voltammetric sensor for sulfamethoxazole. Ceram Int 47:13903–13911
Al-Jawadi AM, Majeed MI (2021) Detection of anticancer drug by electrochemical sensors at modified electrode (MWCNT/polyEosin-Y). Nanomed Res J 6:50–59
Messaoud NB, Ghica ME, Dridi C, Ali MB, Brett CM (2017) Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens Actuators B Chem 253:513–522
Khalilzadeh MA, Tajik S, Beitollahi H, Venditti RA (2020) Green synthesis of magnetic nanocomposite with iron oxide deposited on cellulose nanocrystals with copper (Fe3O4@ CNC/Cu): investigation of catalytic activity for the development of a venlafaxine electrochemical sensor. Indust Eng Chem Res 59:4219–4228
Batista LCD, Santos TIS, Santos JEL, da Silva DR, Martínez-Huitle C (2021) Metal organic Framework-235 (MOF-235) modified carbon paste electrode for catechol determination in water. Electroanalysis 33:57–65
Payehghadr M, Taherkhani Y, Maleki A, Nourifard F (2020) Selective and sensitive voltammetric sensor for methocarbamol determination by molecularly imprinted polymer modified carbon paste electrode. Eurasian Chem Commun 2:982–990
Kalcher K, Kauffmann JM, Wang J, Švancara I, Vytřas K, Neuhold C, Yang Z (1995) Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period 1990–1993. Electroanalysis 7:5–22
Mahmoud AM, Mahnashi MH, El-Wekil MM (2021) Indirect differential pulse voltammetric analysis of cyanide at porous copper based metal organic framework modified carbon paste electrode: application to different water samples. Talanta 221:121562
Abrishamkar M, Ehsani Tilami S, Hosseini Kaldozakh S (2020) Electrocatalytic oxidation of cefixime at the surface of modified carbon paste electrode with synthesized nano zeolite. Adv J Chem A 3:767–776
Payehghadr M, Adineh Salarvand S, Nourifard F, Rofouei MK, Bahramipanah N (2019) Construction of modified carbon paste electrode by a new pantazene ligand for ultra-trace determination of ion silver in real samples. Adv J Chem Section A 2:377–385
Akça A, Karaman O, Karaman C (2021) Mechanistic insights into catalytic reduction of N2O by CO over Cu-embedded graphene: a density functional theory perspective. ECS J Solid State Sci Technol 10:041003
Karimi-Maleh H, Bananezhad A, Ganjali MR, Norouzi P, Sadrnia A (2018) Surface amplification of pencil graphite electrode with polypyrrole and reduced graphene oxide for fabrication of a guanine/adenine DNA based electrochemical biosensors for determination of didanosine anticancer drug. Appl Surf Sci 441:55–60
Karaman C (2021) Orange peel derived-nitrogen and sulfur Co-doped carbon dots: a nano-booster for enhancing ORR electrocatalytic performance of 3D graphene networks. Electroanalysis 33:1356–1369
Karaman C, Karaman O, Atar N, Yola ML (2021) Electrochemical immunosensor development based on core-shell high-crystalline graphitic carbon nitride@carbon dots and Cd0.5Zn0.5S/d-Ti3C2Tx MXene composite for heart-type fatty acid–binding protein detection. Microchim Acta 188:1–15
Tahernejad-Javazmi F, Shabani-Nooshabadi M, Karimi-Maleh H (2019) 3D reduced graphene oxide/FeNi3-ionic liquid nanocomposite modified sensor; an electrical synergic effect for development of tert-butylhydroquinone and folic acid sensor. Compos Part B 172:666–670
Tajik S, Beitollahi H, Jang HW, Shokouhimehr M (2021) A screen printed electrode modified with Fe3O4@polypyrrole-Pt core-shell nanoparticles for electrochemical detection of 6-mercaptopurine and 6-thioguanine. Talanta 232:122379
Shaikh UU, Tamboli Q, Pathange SM, Dahan ZA, Pudukulathan Z (2018) An efficient method for chemoselective acetylation of activated alcohols using nano ZnFe2O4 as catalyst. Chem Methodol 2:73–82
Shahzad H, Ahmadi R, Sheshmani S (2020) Investigating the performance of nano structure C60 as nano-carriers of anticancer cytarabine, a DFT study. Asian J Green Chem 4:355–366
Du H, Xie Y, Wang J (2021) Nanomaterial-sensors for herbicides detection using electrochemical techniques and prospect applications. TrAC Trends Anal Chem 116178.
Khataee A, Sohrabi H, Arbabzadeh O, Khaaki P, Majidi MR (2021) Frontiers in conventional and nanomaterials based electrochemical sensing and biosensing approaches for Ochratoxin A analysis in foodstuffs: a review. Food Chem Toxicol 112030.
Sheikhshoaie I, Rezazadeh A, Ramezanpour S (2018) Removal of Pb (II) from aqueous solution by gel combustion of a new nano sized Co3O4/ZnO composite. Asian J Nanosci Mater 1:271–281
Madadi Z, Soltanieh M, Bagheri Lotfabad T, Nazari S (2019) Green synthesis of titanium dioxide nanoparticles with Glycyrrhiza glabra and their photocatalytic activity. Asian J Green Chem 4:256–268
Ghafour Taher S, Abdulkareem Omar K, Mohammed Faqi-Ahmed B (2019) Green and selective oxidation of alcohols using MnO2 nanoparticles under solvent-free condition using microwave irradiation. Asian J Green Chem 4:231–238
Fazal-ur-Rehman M, Qayyum I (2020) Biomedical scope of gold nanoparticles in medical sciences; an advancement in cancer therapy. J Med Chem Sci 3:399–407
Maleh HK, Orooji Y, Karimi F, Alizadeh M, Baghayeri M, Rouhi J, Tajik S, Beitollahi H, Agarwal S, Gupta VK, Rajendran S, Ayati A, Fu L, Sanati A, Tanhaei B, Sen F, Shabani-nooshabadi M, Asrami PN, Al-Othman A (2021) A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens Bioelectron 184:113252
Karimi-Maleh H, Alizadeh M, Orooji Y, Karimi F, Baghayeri M, Rouhi J, Tajik S, Beitollahi H, Agarwal S, Gupta VK, Rajendran S, Rostamnia S, Fu L, Saberi-Movahed F, Samira M (2021) Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: a docking/experimental investigation. Ind Eng Chem Res 60:816–823
Zhou K, Shen D, Li X, Chen Y, Hou L, Zhang Y, Sha J (2020) Molybdenum oxide-based metal-organic framework/polypyrrole nanocomposites for enhancing electrochemical detection of dopamine. Talanta 209:120507
Dong Y, Yang L, Zhang L (2017) Simultaneous electrochemical detection of benzimidazole fungicides carbendazim and thiabendazole using a novel nanohybrid material-modified electrode. J Agric Food Chem 65:727–736
Haga MA, Kobayashi K, Terada K (2007) Fabrication and functions of surface nanomaterials based on multilayered or nanoarrayed assembly of metal complexes. Coord Chem Rev 251(21–24):2688–2701
Su CC, Jan HC, Kuo-Yih H, Shyh-Jiun L, Shion-Wen W, Sue-Lein W, Sheng-Nan L (1992) Bonding properties of Cu (II)-imidazole chromophores: spectroscopic and electrochemical properties of monosubstituted imidazole copper (II) complexes. Molecular structure of [Cu (4-methylimidazole)4 (ClO4) 2]. Inorg Chim Acta 196:231–240
Kumar P, Joseph A, Ramamurthy PC, Subramanian S (2012) Lead ion sensor with electrodes modified by imidazole-functionalized polyaniline. Microchim Acta 177:317–323
Zhang SS, Niu SY, Qu B, Jie GF, Xu H, Ding CF (2005) Studies on the interaction mechanism between hexakis (imidazole) manganese (II) terephthalate and DNA and preparation of DNA electrochemical sensor. J Inorg Biochem 99:2340–2347
Zhao Z, Zhou B, Su Z, Ma H, Li C (2008) A new [As3Mo3O15] 3− fragment decorated with Cu (I)-imi (imi= imidazole) complexes: Synthesis, structure and electrochemical properties. Inorg Chem Commun 11:648–651
Oberhausen KJ, O’brien RJ, Richardson JF, Buchanan RM (1990) New tripodal Cu (II) complexes containing imidazole ligands. Inorg Chim Acta 173:145–154
Wei X, Li N, Zhang X (2017) Cu@ C nanoporous composites containing little copper oxides derived from dimethyl imidazole modified MOF199 as electrocatalysts for hydrogen evolution reaction. Appl Surf Sci 425:663–673
Forster RJ, Pellegrin Y, Keyes TE (2007) pH effects on the rate of heterogeneous electron transfer across a fluorine doped tin oxide/monolayer interface. Electrochem Commun 9:1899–1906
Liu F, Wang K, Bai G, Zhang Y, Gao L (2004) The pH-induced emission switching and interesting DNA-binding properties of a novel dinuclear ruthenium (II) complex. Inorg Chem 43:1799–1806
Barman K, Jasimuddin S (2014) Electrochemical detection of adenine and guanine using a self-assembled copper (II)–thiophenyl-azo-imidazole complex monolayer modified gold electrode. RSC Adv 4:49819–49826
Neelakantan MA, Sundaram M, Sivasankaran Nair M (2011) Solution equilibria of Ni (II), Cu (II), and Zn (II) complexes involving pyridoxine and imidazole containing ligands: pH metric, spectral, electrochemical, and biological studies. J Chem Eng Data 56:2527–2535
Jain R, Rather JA, Dwivedi A (2010) Highly sensitive and selective voltammetric sensor fullerene modified glassy carbon electrode for determination of cefitizoxime in solubilized system. Electroanalysis 22:2600–2606
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tajik, S., Beitollahi, H., Shahsavari, M. et al. Voltammetric Determination of Ceftizoxime by a Carbon Paste Electrode Modified with Ionic Liquid and Cu (Him)2 Nanoparticles. Top Catal 65, 595–603 (2022). https://doi.org/10.1007/s11244-021-01469-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01469-2