Abstract
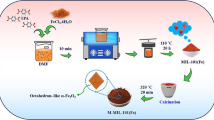
In this report, we focus on the modification of iron terephthalate metal–organic framework (MIL-53(Fe)) by soaking in H2O2 solution and its mechanism. The structure of MIL-53(Fe) before and after the modification were characterized by Fourier-transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), and N2 adsorption–desorption isotherms. The XRD results showed that the material structure changed to amorphous phases and the photocatalytic efficiency was improved after modified by H2O2. The MIL-53 (Fe, H2O2) nanoparticles about 100–300 nm in size was successfully prepared and confirmed by SEM images. In term of UV–Vis DRS results, the absorption spectrum of modified MIL-53(Fe) shifted to higher wavelength and its band gap energy is estimated about 2.2 eV, which is significantly lower than the bandgap value of the conventional material. The impact of the modification on the photocatalytic efficiency was investigated by methylene blue (MB) degradation experiments and photoluminescence (PL) spectroscopy. MB was completely decomposed within 30 min by modified MIL-53(Fe) under optimal conditions. The reaction parameters that affect MB degradation by the as-prepared catalyst were also investigated, including the pH solution, catalyst and H2O2 dosage.











Similar content being viewed by others
References
Yılmaz E, Sert E, Atalay FS (2016) Synthesis, characterization of a metal organic framework: MIL-53 (Fe) and adsorption mechanisms of methyl red onto MIL-53 (Fe). J Taiwan Inst Chem Eng 65:323–330
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res 36:2824–2830
Bennett TD, Cheetham AK (2014) Amorphous metal–organic frameworks. Acc Chem Res 47:1555–1562
Bennett TD, Horike S (2018) Liquid, glass and amorphous solid states of coordination polymers and metal–organic frameworks. Nat Rev Mater 3:431–440
Bennett TD, Keen DA, Tan J-C, Barney ER, Goodwin AL, Cheetham AK (2011) Thermal amorphization of zeolitic imidazolate frameworks. Angew Chem Int Ed 50:3067–3071
Guillerm V, Ragon F, Dan-Hardi M, Devic T, Vishnuvarthan M, Campo B et al (2012) A series of isoreticular, highly stable, porous zirconium oxide based metal–organic frameworks. Angew Chem Int Ed Engl 51:9267–9271
Chapman KW, Sava DF, Halder GJ, Chupas PJ, Nenoff TM (2011) Trapping guests within a nanoporous metal–organic framework through pressure-induced amorphization. J Am Chem Soc 133:18583–18585
Conrad S, Kumar P, Xue F, Ren L, Henning S, Xiao C et al (2018) Controlling dissolution and transformation of zeolitic imidazolate frameworks by using electron-beam-induced amorphization. Angew Chem Int Ed 57:13592–13597
Jiang D, Xu P, Wang H, Zeng G, Huang D, Chen M et al (2018) Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord Chem Rev 376:449–466
Araya T, Jia M, Yang J, Zhao P, Cai K, Ma W et al (2017) Resin modified MIL-53(Fe) MOF for improvement of photocatalytic performance. Appl Catal B 203:768–777
Llewellyn PL, Horcajada P, Maurin G, Devic T, Rosenbach N, Bourrelly S et al (2009) Complex adsorption of short linear alkanes in the flexible metal–organic-framework MIL-53(Fe). J Am Chem Soc 131:13002–13008
El Osta R, Carlin-Sinclair A, Guillou N, Walton RI, Vermoortele F, Maes M et al (2012) Liquid-phase adsorption and separation of xylene isomers by the flexible porous metal–organic framework MIL-53(Fe). Chem Mater 24:2781–2791
Jia J, Xu F, Long Z, Hou X, Sepaniak MJ (2013) Metal-organic framework MIL-53(Fe) for highly selective and ultrasensitive direct sensing of MeHg(+). Chem Commun 49:15
Du JJ, Yuan YP, Sun JX, Peng FM, Jiang X, Qiu LG et al (2011) New photocatalysts based on MIL-53 metal–organic frameworks for the decolorization of methylene blue dye. J Hazard Mater 190:945–951
Hu C, Huang Y-C, Chang A-L, Nomura M (2019) Amine functionalized ZIF-8 as a visible-light-driven photocatalyst for Cr(VI) reduction. J Colloid Interface Sci 553:372–381
Nikseresht A, Daniyali A, Ali-Mohammadi M, Afzalinia A, Mirzaie A (2017) Ultrasound-assisted biodiesel production by a novel composite of Fe(III)-based MOF and phosphotangestic acid as efficient and reusable catalyst. Ultrasonics Sonochem 37:203–207
Trinh ND, Hong SS (2015) Photocatalytic decomposition of methylene blue over MIL-53(Fe) prepared using microwave-assisted process under visible light irradiation. J Nanosci Nanotechnol 15:5450–5454
Dao X-Y, Xie X-F, Guo J-H, Zhang X-Y, Kang Y-S, Sun W-Y (2020) Boosting photocatalytic CO2 reduction efficiency by heterostructures of NH2-MIL-101(Fe)/g-C3N4. ACS Appl Energy Mater 3:3946–3954
Lin R, Li S, Wang J, Xu J, Xu C, Wang J et al (2018) Facile generation of carbon quantum dots in MIL-53(Fe) particles as localized electron acceptors for enhancing their photocatalytic Cr(VI) reduction. Inorg Chem Front 5:3170–3177
Zhang C, Ai L, Jiang J (2015) Solvothermal synthesis of MIL–53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light. J Mater Chem A 3:3074–3081
Liang R, Shen L, Jing F, Qin N, Wu L (2015) Preparation of MIL-53(Fe)-reduced graphene oxide nanocomposites by a simple self-assembly strategy for increasing interfacial contact: efficient visible-light photocatalysts. ACS Appl Mater Interfaces 7:9507–9515
Huang W, Liu N, Zhang X, Wu M, Tang L (2017) Metal organic framework g-C3N4/MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Appl Surf Sci 425:107–116
Xiang W, Zhang Y, Lin H, Liu CJ (2017) Nanoparticle/metal–organic framework composites for catalytic applications: current status and perspective. Molecules 22:30
Cai X, Xie Z, Li D, Kassymova M, Zang S-Q, Jiang H-L (2020) Nano-sized metal–organic frameworks: synthesis and applications. Coord Chem Rev 417:213366
Nguyen M-TH, Nguyen Q-T (2014) Efficient refinement of a metal–organic framework MIL-53(Fe) by UV–Vis irradiation in aqueous hydrogen peroxide solution. J Photochem Photobiol, A 288:55–59
Ai L, Zhang C, Li L, Jiang J (2014) Iron terephthalate metal–organic framework: revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl Catal B 148–149:191–200
He J, Zhang Y, Zhang X, Huang Y (2018) Highly efficient Fenton and enzyme-mimetic activities of NH2-MIL-88B(Fe) metal organic framework for methylene blue degradation. Sci Rep 8:5159
Qu X, Kirschenbaum LJ, Borish ET (2000) Hydroxyterephthalate as a fluorescent probe for hydroxyl radicals: application to hair melanin. Photochem Photobiol 71:307–313
Vu TA, Le GH, Dao CD, Dang LQ, Nguyen KT, Nguyen QK et al (2015) Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv 5:5261–5268
Liang R, Jing F, Shen L, Qin N, Wu L (2015) MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J Hazard Mater 287:364–372
Alvaro M, Carbonell E, Ferrer B, Llabres i Xamena FX, Garcia H (2007) Semiconductor behavior of a metal–organic framework (MOF). Chemistry 13:5106–5112
De Vos A, Hendrickx K, Van Der Voort P, Van Speybroeck V, Lejaeghere K (2017) Missing linkers: an alternative pathway to UiO-66 electronic structure engineering. Chem Mater 29:3006–3019
Wang D, Huang R, Liu W, Sun D, Li Z (2014) Fe-based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways. ACS Catal 4:4254–4260
Chen Q, Wu P, Li Y, Zhu N, Dang Z (2009) Heterogeneous photo-Fenton photodegradation of reactive brilliant orange X-GN over iron-pillared montmorillonite under visible irradiation. J Hazard Mater 168:901–908
Pu M, Guan Z, Ma Y, Wan J, Wang Y, Brusseau ML et al (2018) Synthesis of iron-based metal-organic framework MIL-53 as an efficient catalyst to activate persulfate for the degradation of Orange G in aqueous solution. Appl Catal A 549:82–92
Li X, Guo W, Liu Z, Wang R, Liu H (2016) Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation. Appl Surf Sci 369:130–136
Panda R, Rahut S, Basu JK (2016) Preparation of a Fe2O3/MIL-53(Fe) composite by partial thermal decomposition of MIL-53(Fe) nanorods and their photocatalytic activity. RSC Adv 6:80981–80985
Nguyen V, Nguyen T, Bach L, Hoang T, Bui Q, Tran L et al (2018) Effective photocatalytic activity of mixed Ni/Fe-base metal–organic framework under a compact fluorescent daylight lamp. Catalysts 8:487
Chaturvedi G, Kaur A, Kansal SK (2019) CdS-decorated MIL-53(Fe) microrods with enhanced visible light photocatalytic performance for the degradation of ketorolac tromethamine and mechanism insight. J Phys Chem C 123:16857–16867
Xu X, Li J, Li Y, Ni B, Liu X, Pan L (2018) Chapter 4: selection of carbon electrode materials. In: Ahualli S, Delgado ÁV (eds) Interface Science and Technology. Elsevier, Amsterdam, pp 65–83
Li X, Pi Y, Wu L, Xia Q, Wu J, Li Z et al (2017) Facilitation of the visible light-induced Fenton-like excitation of H2O2 via heterojunction of g-C3N4/NH2-iron terephthalate metal-organic framework for MB degradation. Appl Catal B 202:653–663
Nguyen VH, Bach LG, Bui QTP, Nguyen TD, Vo D-VN, Vu HT et al (2018) Composite photocatalysts containing MIL-53(Fe) as a heterogeneous photo-Fenton catalyst for the decolorization of rhodamine B under visible light irradiation. J Environ Chem Eng 6:7434–7441
Acknowledgements
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under Grant Number 104.01-2014.47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quang, T.T., Truong, N.X., Minh, T.H. et al. Enhanced Photocatalytic Degradation of MB Under Visible Light Using the Modified MIL-53(Fe). Top Catal 63, 1227–1239 (2020). https://doi.org/10.1007/s11244-020-01364-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01364-2