Abstract
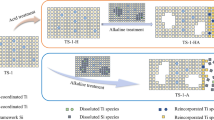
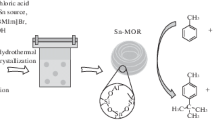
A series of modified TS-1 catalysts was prepared by post-synthesis hydrothermal treatment of a parent TS-1 zeolite with solutions of different concentrations of tetrapropylammonium hydroxide (TPAOH). The treatment results in an advantageous improvement of the catalytic activity for the liquid oxidation of furfural with H2O2 to produce maleic acid. The highest yield of maleic acid (83%) was obtained when using 0.025 M TPAOH solution; while the maximum yield of the untreated TS-1 was 70%. The catalysts were thoroughly characterised by XRD, UV–Vis, ICP–OES, XPS, TEM, N2-adsorption, DRIFT of chemisorbed deuterated acetonitrile and TEM, in order to elucidate the origin of the catalytic improvement. The characterisation studies allowed us to conclude that, besides the well-known creation of mesopores within the zeolite primary particles, the TPAOH treatment also results in the increase of the hydrophobicity balance of the channels and cavities of the zeolites (via silanols removal). Both properties have a relevant effect on the improvement of the catalytic properties.








Similar content being viewed by others
References
Felthouse TR, Horrell JCB,B, Mummey MJ, Kuo Y-J (2001) Maleic anhydride, maleic acid and fumaric acid. In: Othmer D, Kirk RE (eds) Kirk-Othmer Encyclopedia of Chemical Technology Wiley, Hoboken
Lohbeck K, Fuhrmann HH,W, Fedtke N (2000) Maleic and Fumaric Acids. In: Campbell FT, Pfefferkorn R, Rounsaville JF (eds) Ullmann´s Encyclopedia of Industrial Chemistry Weinheim, Germany vol 20. pp 463–473
Pavarelli G, Velasquez Ochoa J, Caldarelli A, Puzzo F, Cavani F, Dubois J-L (2015) A new process for maleic anhydride synthesis from a renewable building block: the gas-phase oxidehydration of Bio-1-butanol. ChemSusChem 8(13):2250–2259. https://doi.org/10.1002/cssc.201500095
Chatzidimitriou A, Bond JQ (2015) Oxidation of levulinic acid for the production of maleic anhydride: breathing new life into biochemicals. Green Chem 17(8):4367–4376. https://doi.org/10.1039/C5GC01000D
Du Z, Ma J, Wang F, Liu J, Xu J (2011) Oxidation of 5-hydroxymethylfurfural to maleic anhydride with molecular oxygen. Green Chem 13(3):554–557. https://doi.org/10.1039/C0GC00837K
Xia C, Lin M, Zheng A, Xiang Y, Zhu B, Xu G, Shu X (2016) Irreversible deactivation of hollow TS-1 zeolite caused by the formation of acidic amorphous TiO2–SiO2 nanoparticles in a commercial cyclohexanone ammoximation process. J Catal 338:340–348. https://doi.org/10.1016/j.jcat.2016.02.032
Lan J, Lin J, Chen Z, Yin G (2015) Transformation of 5-hydroxymethylfurfural (HMF) to maleic anhydride by aerobic oxidation with heteropolyacid catalysts. ACS Catal 5(4):2035–2041. https://doi.org/10.1021/cs501776n
Lv G, Chen C, Lu B, Li J, Yang Y, Chen C, Deng T, Zhu Y, Hou X (2016) Vanadium-oxo immobilized onto Schiff base modified graphene oxide for efficient catalytic oxidation of 5-hydroxymethylfurfural and furfural into maleic anhydride. RSC Adv 6(103):101277–101282. https://doi.org/10.1039/C6RA21795H
Alonso-Fagúndez N, Laserna V, Alba-Rubio AC, Mengibar M, Heras A, Mariscal R, Granados ML (2014) Poly-(styrene sulphonic acid): an acid catalyst from polystyrene waste for reactions of interest in biomass valorization. Catal Today 234:285–294. https://doi.org/10.1016/j.cattod.2014.01.041
Guo H, Yin G (2011) Catalytic aerobic oxidation of renewable furfural with phosphomolybdic acid catalyst: an alternative route to maleic acid. J Phys Chem C 115(35):17516–17522. https://doi.org/10.1021/jp2054712
Shi S, Guo H, Yin G (2011) Synthesis of maleic acid from renewable resources: catalytic oxidation of furfural in liquid media with dioxygen. Catal Commun 12(8):731–733. https://doi.org/10.1016/j.catcom.2010.12.033
Alba-Rubio AC, Fierro JLG, León-Reina L, Mariscal R, Dumesic JA, López Granados M (2017) Oxidation of furfural in aqueous H2O2 catalysed by titanium silicalite: deactivation processes and role of extraframework Ti oxides. Appl Catal B 202:269–280. https://doi.org/10.1016/j.apcatb.2016.09.025
Lan J, Chen Z, Lin J, Yin G (2014) Catalytic aerobic oxidation of renewable furfural to maleic anhydride and furanone derivatives with their mechanistic studies. Green Chem 16(9):4351–4358. https://doi.org/10.1039/C4GC00829D
Choudhary H, Nishimura S, Ebitani K (2013) Metal-free oxidative synthesis of succinic acid from biomass-derived furan compounds using a solid acid catalyst with hydrogen peroxide. Appl Catal A 458:55–62. https://doi.org/10.1016/j.apcata.2013.03.033
Hemant C, Shun N, Kohki E (2012) Highly efficient aqueous oxidation of furfural to succinic acid using reusable heterogeneous acid catalyst with hydrogen peroxide. Chem Lett 41(4):409–411. https://doi.org/10.1246/cl.2012.409
Li X, Lan X, Wang T (2016) Selective oxidation of furfural in a bi-phasic system with homogeneous acid catalyst. Catal Today 276:97–104. https://doi.org/10.1016/j.cattod.2015.11.036
Huang Y, Wu C, Yuan W, Xia Y, Liu X, Yang H, Wang H (2017) Catalytic aerobic oxidation of biomass-based furfural into maleic acid in aqueous phase with metalloporphyrin catalysts. J Chin Chem Soc 64(7):786–794. https://doi.org/10.1002/jccs.201700004
Alonso-Fagundez N, Agirrezabal-Telleria I, Arias PL, Fierro JLG, Mariscal R, Granados ML (2014) Aqueous-phase catalytic oxidation of furfural with H2O2: high yield of maleic acid by using titanium silicalite-1. RSC Adv 4(98):54960–54972. https://doi.org/10.1039/C4RA11563E
Alonso-Fagúndez N, Ojeda M, Mariscal R, Fierro JLG, López Granados M (2017) Gas phase oxidation of furfural to maleic anhydride on V2O5/γ-Al2O3 catalysts: reaction conditions to slow down the deactivation. J Catal 348:265–275. https://doi.org/10.1016/j.jcat.2016.12.005
Mamman AS, Lee J-M, Kim Y-C, Hwang IT, Park N-J, Hwang YK, Chang J-S, Hwang J-S (2008) Furfural: hemicellulose/xylosederived biochemical. Biofuels Bioprod Biorefin 2(5):438–454. https://doi.org/10.1002/bbb.95
Karinen R, Vilonen K, Niemelä M (2011) Biorefining: heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. ChemSusChem 4(8):1002–1016. https://doi.org/10.1002/cssc.201000375
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem 12(4):539–554. https://doi.org/10.1039/B922014C
Mariscal R, Maireles-Torres P, Ojeda M, Sadaba I, Lopez Granados M (2016) Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9(4):1144–1189. https://doi.org/10.1039/C5EE02666K
Alonso-Fagúndez N, Granados ML, Mariscal R, Ojeda M (2012) Selective conversion of furfural to maleic anhydride and furan with VOx/Al2O3 catalysts. ChemSusChem 5(10):1984–1990. https://doi.org/10.1002/cssc.201200167
Halasz I, Agarwal M, Senderov E, Marcus B (2003) Continuous monitoring the oxyfunctionalization of hexane by aqueous H2O2 over TS-1 related catalysts. Appl Catal A 241(1):167–184. https://doi.org/10.1016/S0926-860X(02)00464-7
Clerici MG (2015) The activity of titanium silicalite-1 (TS-1): some considerations on its origin. Kinet Catal 56(4):450–455. https://doi.org/10.1134/s0023158415040059
Wu G, Lin Z, Li L, Zhang L, Hong Y, Wang W, Chen C, Jiang Y, Yan X (2017) Experiments and kinetics of the epoxidation of allyl chloride with H2O2 over organic base treated TS-1 catalysts. Chem Eng J 320(Supplement C):1–10. https://doi.org/10.1016/j.cej.2017.03.030
Tsai S-T, Chao P-Y, Tsai T-C, Wang I, Liu X, Guo X-W (2009) Effects of pore structure of post-treated TS-1 on phenol hydroxylation. Catal Today 148(1):174–178. https://doi.org/10.1016/j.cattod.2009.02.018
Wang Y, Lin M, Tuel A (2007) Hollow TS-1 crystals formed via a dissolution–recrystallization process. Microporous Mesoporous Mater 102(1):80–85. https://doi.org/10.1016/j.micromeso.2006.12.019
Xue Y, Wen Y, Wei H, Liu M, Huang X, Ye X, Wang X, Li B (2015) Hollow TS-1 mesocrystals: hydrothermal construction and high catalytic performances in cyclohexanone ammoximation. RSC Adv 5(64):51563–51569. https://doi.org/10.1039/C5RA05999B
Kulawika K, Schulz-Ekloff G, Rathouský J, Zukal A, Had J (1995) Hydroxylation of phenol over Ti-MCM-41 and TS-1. Collect Czechoslov Chem Commun 60(3):451
Wang Y, Liu W, Lin Y, Ye J, Wang S, Li H (2016) Effects of the amount of tetrapropyl ammonium hydroxide in synthesis on TS-1 properties and catalytic performance in epoxidation of propylene. Trans Tianjin Univ 22(5):458–465. https://doi.org/10.1007/s12209-016-2780-1
Rodenas Y, Mariscal R, Fierro JLG, Martin Alonso D, Dumesic JA, Lopez Granados M (2018) Improving the production of maleic acid from biomass: TS-1 catalysed aqueous phase oxidation of furfural in the presence of [gamma]-valerolactone. Green Chem 20(12):2845–2856. https://doi.org/10.1039/C8GC00857D
Kumar P, Pandey RK (2000) An efficient synthesis of 5-hydroxy-2 (5H)-furanone using a titanium silicate molecular sieve catalyst. Green Chem 2:29–31
Wagner CD (1983) Sensitivity factors for XPS analysis of surface atoms. J Electron Spectrosc Relat Phenom 32(2):99–102. https://doi.org/10.1016/0368-2048(83)85087-7
Vayssilov GN (1997) Structural and physicochemical features of titanium silicalites. Catal Rev 39(3):209–251. https://doi.org/10.1080/01614949709353777
Bonino F, Damin A, Ricchiardi G, Ricci M, Spanò G, D’Aloisio R, Zecchina A, Lamberti C, Prestipino C, Bordiga S (2004) Ti-peroxo species in the TS-1/H2O2/H2O system. J Phys Chem B 108(11):3573–3583. https://doi.org/10.1021/jp036166e
Wahlen J, Moens B, De Vos DE, Alsters PL, Jacobs PA (2004) Titanium silicalite 1 (TS-1) catalyzed oxidative transformations of furan derivatives with hydrogen peroxide. Adv Synth Catal 346(2–3):333–338. https://doi.org/10.1002/adsc.200303185 doi
Guo Q, Sun K, Feng Z, Li G, Guo M, Fan F, Li C (2012) A thorough investigation of the active titanium species in TS-1 zeolite by in situ UV resonance Raman spectroscopy. Chem Eur J 18(43):13854–13860. https://doi.org/10.1002/chem.201201319 doi
Groen JC, Peffer LAA, Pérez-Ramírez J (2003) Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater 60(1):1–17. https://doi.org/10.1016/S1387-1811(03)00339-1
Mariscal R, López-Granados M, Fierro JLG, Sotelo JL, Martos C, Van Grieken R (2000) Morphology and surface properties of titania–silica hydrophobic xerogels. Langmuir 16(24):9460–9467. https://doi.org/10.1021/la000876j
Bonino F, Damin A, Bordiga S, Lamberti C, Zecchina A (2003) Interaction of CD3CN and pyridine with the Ti(IV) centers of TS-1 catalysts: a spectroscopic and computational study. Langmuir 19(6):2155–2161. https://doi.org/10.1021/la0262194
Zecchina A, Bordiga S, Spoto G, Marchese L, Petrini G, Leofanti G, Padovan M (1992) Silicalite characterization. 2. IR spectroscopy of the interaction of carbon monoxide with internal and external hydroxyl groups. J Phys Chem 96(12):4991–4997. https://doi.org/10.1021/j100191a048
Acknowledgements
Financial support from the Spanish Ministry of Science, Innovation and Universities (MICINN) (project CTQ2015-64226-C3-1-R) and from CSIC (i-link1048 project) is gratefully acknowledged. Y.R. thanks MINECO for her FPI pre-doctoral grant (BES-2016-077184) and M.R. thanks the MINECO project ENE2016-77055-C3-3-R for her postdoctoral contract.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodenas, Y., Fierro, J.L.G., Mariscal, R. et al. Post-synthesis Treatment of TS-1 with TPAOH: Effect of Hydrophobicity on the Liquid-Phase Oxidation of Furfural to Maleic Acid. Top Catal 62, 560–569 (2019). https://doi.org/10.1007/s11244-019-01149-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-019-01149-2