Abstract
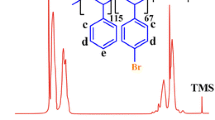
Crystalline syndiotactic polystyrene (sPS) was functionalized by superacid-catalyzed Friedel–Crafts alkylation. Once a tertiary carbocation is generated by the reaction of tertiary alcohols and triflic acid, it subsequently reacted with aromatic ring of sPS, forming alkyl functionalized sPS. Various functional groups were incorporated into the phenyl ring of sPS using different substrates of tertiary alcohols including tertiary butanol, 2-methyl-2-butanol, and 2-methyl-2-hexanol. Degree of functionalization was conveniently tunable by changing the ratio of tertiary alcohol to styrene unit, and a maximum 80% of styrene rings was functionalized. Thermal properties of the functionalized sPSs were investigated by differential scanning calorimetry. Glass transition temperature and melting temperature of sPS were reduced after the modification.





Similar content being viewed by others
References
Malanga M, Isogai O, Yamada T, Iwasaki S, Kuramoto M (2009) Historical overview and commercialization of syndiotactic polystyrene. In: Syndiotactic polystyrene. Wiley, New York, pp 1–13
Ishihara N, Kuramoto M, Uoi M (1988) Stereospecific polymerization of styrene giving the syndiotactic polymer. Macromolecules 21:3356–3360
Ishihara N, Seimiya T, Kuramoto M, Uoi M (1986) Crystalline syndiotactic polystyrene. Macromolecules 19:2464–2465
Ishihara N (1995) Syntheses and properties of syndiotactic polystyrene. Macromol Symp 89:553–562
Zambelli A, Oliva L, Pellecchia C (1989) Soluble catalysts for syndiotactic polymerization of styrene. Macromolecules 22:2129–2130
Kaminsky W (1998) Highly active metallocene catalysts for olefin polymerization. J Chem Soc J Chem Soc Dalton Trans 0:1413–1418
Zinck P, Bonnet F, Mortreux A, Visseaux M (2009) Functionalization of syndiotactic polystyrene. Prog Polym Sci 34:369–392
Jaymand M (2014) Recent progress in the chemical modification of syndiotactic polystyrene. Polym Chem 5:2663–2690
Soga K, Nakatani H, Monoi T (1990) Copolymerization of styrene and substituted styrenes with tetramenthoxytitanium-methylaluminoxane catalyst. Macromolecules 23:953–957
Xu G, Chung TC (2000) Synthesis of syndiotactic polystyrene derivatives containing amino groups. Macromolecules 33:5803–5809
Dong JY, Manias E, Chung TC (2002) Functionalized syndiotactic polystyrene polymers prepared by the combination of metallocene catalyst and borane comonomer. Macromolecules 35:3439–3447
Wallace RA (1971) Glass transition in partially sulfonated polystyrene. J Polym Sci Part A-2 Polym Phys 9:1325–1332
Weiss RA, Agarwal PK, Lundberg RD (1984) Control of ionic interactions in sulfonated polystyrene ionomers by the use of alkyl-substituted ammonium counterions. J Appl Polym Sci 29:2719–2734
Coughlin JE, Reisch A, Markarian MZ, Schlenoff JB (2013) Sulfonation of polystyrene: toward the “ideal” polyelectrolyte. J Polym Sci Part A Polym Chem 51:2416–2424
Orler EB, Yontz DJ, Moore RB (1993) Sulfonation of syndiotactic polystyrene for model semicrystalline ionomer investigations. Macromolecules 26:5157–5160
Orler EB, Moore RB (1994) Influence of ionic interactions on the crystallization of lightly sulfonated syndiotactic polystyrene ionomers. Macromolecules 27:4774–4780
Fahs GB, Benson SD, Moore RB (2017) Blocky sulfonation of syndiotactic polystyrene: a facile route toward tailored ionomer architecture via postpolymerization functionalization in the gel state. Macromolecules 50:2387–2396
Gao Y, Li H-M (2004) Synthesis and characterization of acetylated syndiotactic polystyrene. Polym Int 53:1436–1441
Gao Y, Li H-M, Liu F-S, Wang X-Y, Shen Z-G (2007) Synthesis and characterization of benzoylated syndiotactic polystyrene. J Polym Res 14:291–296
Li J, Li H-M (2005) Functionalization of syndiotactic polystyrene with succinic anhydride in the presence of aluminum chloride. Eur Polym J 41:823–829
Shin J, Jensen SM, Ju J, Lee S, Xue Z, Noh SK, Bae C (2007) Controlled functionalization of crystalline polystyrenes via activation of aromatic C–H bonds. Macromolecules 40:8600–8608
Shin J, Chang Y, Nguyen TLT, Noh SK, Bae C (2010) Hydrophilic functionalization of syndiotactic polystyrene via a combination of electrophilic bromination and Suzuki–Miyaura reaction. J Polym Sci Part A Polym Chem 48:4335–4343
Jeon JY, Mohanty AD, Tian D, Bae C (2017) Ionic functionalization of polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene via Friedel-Crafts bromoalkylation and its application for anion exchange membranes. ECS Trans 80:967–970
Klumpp DA, Baek DN, Prakash GKS, Olah GA (1997) Preparation of condensed aromatics by superacidic dehydrative cyclization of aryl pinacols and epoxides1a. J Org Chem 62:6666–6671
Klumpp DA, Fredrick S, Lau S, Jin KK, Bau R, Surya Prakash GK, Olah GA (1999) Acid-catalyzed condensations of ninhydrin with aromatic compounds. Preparation of 2,2-diaryl-1,3-indanediones and 3-(diarylmethylene)isobenzofuranones1. J Org Chem 64:5152–5155
Pasztor AJ, Landes BG, Karjala PJ (1991) Thermal properties of syndiotactic polystyrene. Thermochim Acta 177:187–195
De Rosa C, Ruiz de Ballesteros O, Di Gennaro M, Auriemma F (2003) Crystallization from the melt of α and β forms of syndiotactic polystyrene. Polymer 44:1861–1870
Guerra G, Vitagliano VM, De Rosa C, Petraccone V, Corradini P (1990) Polymorphism in melt crystallized syndiotactic polystyrene samples. Macromolecules 23:1539–1544
Chow TS (1980) Molecular interpretation of the glass transition temperature of polymer-diluent systems. Macromolecules 13:362–364
Lodge TP, McLeish TCB (2000) Self-concentrations and effective glass transition temperatures in polymer blends. Macromolecules 33:5278–5284
Acknowledgements
Financial support from National Science Foundation (CHE 1534355) and U.S. Department of Energy (ARPA-E, IONICS DE-FOA-0001478) is greatly appreciated for C.B. G.N.K and S.L. also acknowledge the donors of the American Chemical Society Petroleum Research Fund for partial support of this research. The authors thank LG Chem Ltd. for providing sPS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to our colleague George A. Olah who pioneered acid-catalyzed organic reactions using superacid.
Rights and permissions
About this article
Cite this article
Jeon, J.Y., Umstead, Z., Kangovi, G.N. et al. Functionalization of Syndiotactic Polystyrene via Superacid-Catalyzed Friedel–Crafts Alkylation. Top Catal 61, 610–615 (2018). https://doi.org/10.1007/s11244-018-0913-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-0913-6