Abstract
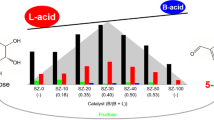
The catalytic performance of two types of heterogeneous acid catalysts—sulfonic acid-functionalized materials and aluminum containing zeolites,—in the dehydration of sorbitol to isosorbide, in solventless and autogenous pressure conditions, has been studied. Catalysts screening evidenced strong differences between sulfonic acid-based materials and acid zeolites in terms of catalytic performance. Whereas sulfonic materials, such as Amberlyst-70 and SBA-15-Pr-SO3H, showed a very high catalytic activity, zeolites with beta structure evidenced good catalytic performance together with minimized promotion of side reactions (production of non-desired sorbitans, humins, etc.). Kinetic studies performed at different temperatures, adjusting to a Langmuir–Hinshelwood type model, allowed correlating the physicochemical properties of the acid materials with their catalytic performance in sorbitol dehydration. Thus, the analysis of initial selectivity through kinetic constants comparison indicated that commercial beta zeolite with a Si/Al ratio of 19 is the most selective catalyst for the production of isosorbide, though following a slower kinetics than the sulfonic materials. Furthermore, an equivalent hierarchical beta zeolite has been synthesised and evaluated, resulting in a slight improvement of the catalytic performance, in terms of both yield and selectivity to isosorbide. This improvement is attributed to the superior textural properties.







Similar content being viewed by others
References
Fukuoka A, Dhepe PL (2006) Angew Chem Int Ed 118:5285–5287
Van de Vyver S, Geboers J, Jacobs PA, Sels BF (2011) ChemCatChem 3:82–94
Gallezot P, Cerino PJ, Blanc B, Flèche G, Fuertes P (1994) J Catal 146:93–102
Hoffer BW, Crezee E, Mooijman PRM, van Langeveld AD, Kapteijn F, Moulijn JA (2003) Catal Today 79–80:35–41
Zhang B, Li X, Wu Q, Zhang C, Yu Y, Lan M, Wei X, Ying Z, Liu T, Liang G, Zhao F (2016) Green Chem 18:3315–3323
Faba F, Kusema BT, Murzina EV, Tokarev A, Kumar N, Smeds A, Díaz E, Ordóñez S, Mäki-Arvela P, Willför S, Salmi T, Murzin DY (2014) Microporous Mesoporous Mater 189:189–199
Zhang J, Li J-B, Wu S-B, Liu Y (2013) Ind Eng Chem Res 52:11799–11815
Rose M, Palkovits R (2012) ChemSusChem 5:167–176
Fenouillot F, Rousseau A, Colomines G, Saint-Loup R, Pascault J-P (2010) Progr Polym Sci 35:578–622
Sheldon RA (2014) Green Chem 16:950–963
Flèche G, Huchette M (1986) Starch/Stärke 38(1):26–30
Bock K, Pedersen P, Thogersen H (1981) Acta Chem Scand B 35:441–449
Li N, Huber GW (2010) J Catal 270:48–59
Yang G, Pidko EA, Hensen EJM (2012) J Catal 295:122–132
Polaert I, Felix MC, Fornasero M, Marcotte S, Buvat J-C, Estel L (2013) Chem Eng J 222:228–239
Li J, Buijs W, Berger RJ, Moulijn JA, Makkle M (2014) Catal Sci Technol 4:152–163
Haines AH, Wells AG (1973) Carbohydr Res 27:261–264
Koerner TAW, Voll RJ, Younathan ES (1977) Carbohydr Res 59:403–416
Yabushita M, Kobayashi H, Shrotri A, Hara K, Ito S, Fukuoka A (2015) Bull Chem Soc Jpn 88:996–1002
Kurszewska M, Skorupowa E, Madaj J, Konitz A, Wojnowki W, Wiśniewski A (2002) Carbohydr Res 337:1261–1268
Kobayashi H, Yokoyama H, Feng B, Fukuoka A (2015) Green Chem 17:2732–2735
Otomo R, Yokoi T, Tatsumi T (2015) Appl Catal A 505:28–35
Yamaguchi A, Sato O, Mimura N, Shirai M (2015) Catal Commun 67:59–63
Barbaro P, Liguori F, Moreno-Marrodan C (2016) Green Chem 18:2935–2940
Kang HY, Hwang DW, Hwang YK, Hwang J-S, Chang J-S (2013) Korean Chem Eng Res 51:189–194
Cubo A, Iglesias J, Morales G, Melero JA, Moreno J, Sánchez-Vázquez R (2017) Appl Catal A. doi:10.1016/j.apcata.2016.10.029
Dabbawala AA, Park JJ, Valekar AH, Mishra DK, Hwang J-S (2015) Catal Commun 69:207–211
Shi J, Shan Y, Tian Y, Wan Y, Zheng Y, Feng Y (2016) RSC Adv 6:13514–13521
Xia J, Yu D, Hu Y, Zou B, Sun P, Li H, Huang H (2011) Catal Commun 12:544–547
Khan NA, Mishra DK, Ahmed I, Yoon JW, Hwang J-S, Jhung SH (2013) Appl Catal A 452:34–38
Ahmed I, Khan NA, Mishra DK, Lee JS, Hwang J-S, Jhung SH (2013) Chem Eng Sci 93:91–95
Dabbawala AA, Mishra DK, Hwang JS (2013) Catal Commun 42:1–5
Rusu OA, Hoelderich WF, Wyart H, Ibert M (2015) Appl Catal B 176–177:139–149
Zhang J, Wang L, Liu F, Meng X, Mao J, Xiao F-S (2014) Catal Today 242:249–254
Dabbawala AA, Mishra DK, Huber GW, Hwang J-S (2015) Appl Catal A 492:252–261
Margolese D, Melero JA, Christiansen SC, Chmelka B, Stucky GD (2000) Chem Mater 12(8):2448–2459
Serrano DP, Aguado J, Escola JM, Rodríguez JM, Peral A (2006) Chem Mater 18(10):2462–2464
Ruiz-Matute AI, Hernández-Hernández O, Rodríguez-Sánchez S., Sanz ML, Martínez-Castro I (2011) J Chromatogr B 879:1226–1240
Schummer C, Delhomme O, Appenzeller BMR, Wenning R, Millet M (2009) Talanta 77:1473–1482
Melero JA, Stucky GD, van Grieken R, Morales G (2002) J Mater Chem 12:1–8
Melero JA, Iglesias J, Morales G (2013) Designing porous inorganic architectures. In: Wilson K, Lee AF (eds) Heterogeneous catalysts for clean technology: spectroscopy, design, and monitoring. Wiley, Weinheim. doi:10.1002/9783527658985.ch8
Acknowledgements
Financial support from Spanish Ministry of Economy and Competitiveness (Project CTQ2014-52907-R) and from the Regional Government of Madrid (Project S2013/MAE-2882) is kindly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morales, G., Iglesias, J., Melero, J.A. et al. Isosorbide Production from Sorbitol over Heterogeneous Acid Catalysts: Screening and Kinetic Study. Top Catal 60, 1027–1039 (2017). https://doi.org/10.1007/s11244-017-0794-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0794-0