Abstract
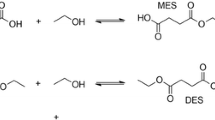
Hydrophobic formic acid esters have been established as alternative cosubstrates for the formate dehydrogenase (FDH)-catalyzed regeneration of reduced nicotinamides. With this approach challenges related to the ionic nature of the commonly used formate salts, particularly their exclusive water-solubility, can be overcome. Octyl formate was demonstrated to serve as organic phase solubilizing hydrophobic reagents as well as serving as a source of reducing equivalents to enable FDH-catalyzed regeneration of NADH. This system was used to drive a monooxygenase-catalyzed hydroxylation reaction. Phase transfer limitations appear to be the overall rate-limitation of the biphasic reaction system.





Similar content being viewed by others
References
Weckbecker A, Groger H, Hummel W (2010) Regeneration of nicotinamide coenzymes: principles and applications for the synthesis of chiral compounds. In: Wittmann C, Krull WR (eds) Biosystems engineering I: creating superior biocatalysts, vol 120. Advances in biochemical engineering-biotechnology. Springer, Berlin, pp 195–242. doi:10.1007/10_2009_55
van der Donk WA, Zhao H (2003) Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol 14(4):421–426
Hollmann F, Arends IWCE, Holtmann D (2011) Enzymatic reductions for the chemist. Green Chem 13(9):2285–2313
Hollmann F, Arends IWCE, Buehler K, Schallmey A, Buhler B (2011) Enzyme-mediated oxidations for the chemist. Green Chem 13:226–265
Hollmann F, Arends Isabel WCE, Buehler K (2010) Biocatalytic redox reactions for organic synthesis: nonconventional regeneration methods. ChemCatChem 2(7):762–782
Rodriguez C, Lavandera I, Gotor V (2012) Recent advances in cofactor regeneration systems applied to biocatalyzed oxidative processes. Curr Org Chem 16(21):2525–2541. doi:10.2174/138527212804004643
Shaked Z, Whitesides GM (1980) Enzyme-catalyzed organic synthesis—NADH regeneration by using formate dehydrogenase. J Am Chem Soc 102(23):7104–7105. doi:10.1021/ja00543a038
Sonoike S, Itakura T, Kitamura M, Aoki S (2012) One-pot chemoenzymatic synthesis of chiral 1,3-diols using an enantioselective aldol reaction with chiral Zn2+ complex catalysts and enzymatic reduction using oxidoreductases with cofactor regeneration. Chem-Asian J 7(1):64–74. doi:10.1002/asia.201100584
Chen Y, Goldberg SL, Hanson RL, Parker WL, Gill I, Tully TP, Montana MA, Goswami A, Patel RN (2011) Enzymatic preparation of an (S)-amino acid from a racemic amino acid. Org Proc Res Dev 15(1):241–248. doi:10.1021/op1001534
Kuehnel K, Maurer SC, Galeyeva Y, Frey W, Laschat S, Urlacher VB (2007) Hydroxylation of dodecanoic acid and (2R,4R,6R,8R)-tetramethyldecanol on a preparative scale using an NADH-dependent CYP102A1 mutant. Adv Synth Catal 349(8–9):1451–1461. doi:10.1002/adsc.200700054
Maurer SC, Schulze H, Schmid RD, Urlacher VB (2003) Immobilisation of P450 BM-3 and an NADP+ cofactor recycling system: towards a technical application of heme-containing monooxygenases in fine chemical synthesis. Adv Synth Catal 345(6–7):802–810
Hofstetter K, Lutz J, Lang I, Witholt B, Schmid A (2004) Coupling of biocatalytic asymmetric epoxidation with nadh regeneration in organic-aqueous emulsions. Angew Chem Int Ed 43(16):2163–2166
Lutz J, Mozhaev VV, Khmelnitsky YL, Witholt B, Schmid A (2002) Preparative application of 2-hydroxybiphenyl 3-monooxygenase with enzymatic cofactor regeneration in organic-aqueous reaction media. J Mol Catal B Enzym 19–20:177–187
Schmid A, Vereyken I, Held M, Witholt B (2001) Preparative regio- and chemoselective functionalization of hydrocarbons catalyzed by cell free preparations of 2-hydroxybiphenyl 3-monooxygenase. J Mol Catal B Enzym 11(4–6):455–462
Eckstein M, Villela M, Liese A, Kragl U (2004) Use of an ionic liquid in a two-phase system to improve an alcohol dehydrogenase catalysed reduction. Chem Commun 9:1084–1085. doi:10.1039/b401065e
Jakoblinnert A, Mladenov R, Paul A, Sibilla F, Schwaneberg U, Ansorge-Schumacher MB, de Maria PD (2011) Asymmetric reduction of ketones with recombinant E. coli whole cells in neat substrates. Chem Commun 47(44):12230–12232
Churakova E, Arends IWCE, Hollmann F (2013) Increasing the productivity of peroxidase-catalyzed oxyfunctionalization: a case study on the potential of two-liquid-phase systems. ChemCatChem 5:565–568. doi:10.1002/cctc.201200490
Frohlich P, Albert K, Bertau M (2011) Formate dehydrogenase—a biocatalyst with novel applications in organic chemistry. Org Biomol Chem 9(22):7941–7950
Hollmann F, Schmid A, Steckhan E (2001) The first synthetic application of a monooxygenase employing indirect electrochemical NADH regeneration. Angew Chem Int Ed 40(1):169–171
Held M, Schmid A, Kohler HPE, Suske W, Witholt B, Wubbolts MG (1999) An integrated process for the production of toxic catechols from toxic phenols based on a designer biocatalyst. Biotechnol Bioeng 62(6):641–648
Held M, Suske W, Schmid A, Engesser KH, Kohler HPE, Witholt B, Wubbolts MG (1998) Preparative scale production of 3-substituted catechols using a novel monooxygenase from Pseudomonas azelaica HBP 1. J Mol Catal B Enzym 5(1–4):87–93
Suske WA, Held M, Schmid A, Fleischmann T, Wubbolts MG, Kohler H-PE (1997) Purification and characterization of 2-hydroxybiphenyl 3-monooxygenase, a novel NADH-dependent, FAD-containing aromatic hydroxylase from Pseudomonas azelaica HBP1. J Biol Chem 272(39):24257–24265. doi:10.1074/jbc.272.39.24257
Schmid A, Kohler HPE, Engesser KH (1998) E-coli JM109 pHBP461, a recombinant biocatalyst for the regioselective monohydroxylation of ortho-substituted phenols to their corresponding 3-substituted catechols. J Mol Catal B Enzym 5(1–4):311–316
Slusarczyk H, Felber S, Kula MR, Pohl M (2000) Stabilization of NAD-dependent formate dehydrogenase from Candida boidinii by site-directed mutagenesis of cysteine residues. Eur J Biochem 267(5):1280–1289. doi:10.1046/j.1432-1327.2000.01123.x
Suske WA, van Berkel WJH, Kohler H-PE (1999) Catalytic mechanism of 2-hydroxybiphenyl 3-monooxygenase, a flavoprotein from Pseudomonas azelaica HBP1. J Biol Chem 274(47):33355–33365. doi:10.1074/jbc.274.47.33355
Bornscheuer U, Kazlauskas R (2006) Hydrolases in organic synthesis, 2nd edn. Wiley, Weinheim
Musa MM, Phillips RS (2011) Recent advances in alcohol dehydrogenase-catalyzed asymmetric production of hydrophobic alcohols. Catal Sci Technol 1(8):1311–1323. doi:10.1039/c1cy00160d
Thorey P, Knez Z, Habulin M (2010) Alcohol dehydrogenase in non-aqueous media using high-pressure technologies: reaction set-up and deactivation determination. J Chem Technol Biotechnol 85(7):1011–1016. doi:10.1002/jctb.2411
Lavandera I, Kern A, Schaffenberger M, Gross J, Glieder A, de Wildeman S, Kroutil W (2008) An exceptionally DMSO-tolerant alcohol dehydrogenase for the stereoselective reduction of ketones. ChemSusChem 1(5):431–436. doi:10.1002/cssc.200800032
Orlich B, Berger H, Lade M, Schomacker R (2000) Stability and activity of alcohol dehydrogenases in W/O-microemulsions: enantioselective reduction including cofactor regeneration. Biotechnol Bioeng 70(6):638–646
Matsuda T, Harada T, Nakamura K (2000) Alcohol dehydrogenase is active in supercritical carbon dioxide. Chem Comm 23(15):1367–1368
Grunwald J, Wirz B, Scollar MP, Klibanov AM (1986) Asymmetric oxidoreductions catalyzed by alcohol dehydrogenase in organic solvents. J Am Chem Soc 108(21):6732–6734. doi:10.1021/ja00281a044
Klibanov AM (2003) Asymmetric enzymatic oxidoreductions in organic solvents. Curr Opin Biotechnol 14(4):427–431
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409(6817):241–246
Acknowledgments
This Project was supported by the BIOTRAINS Marie Curie Initial Training Network, financed by the European Union through the 7th Framework People Programme (Grant agreement number 238531).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ekaterina Churakova and Bartłomiej Tomaszewski contributed equally.
Rights and permissions
About this article
Cite this article
Churakova, E., Tomaszewski, B., Buehler, K. et al. Hydrophobic Formic Acid Esters for Cofactor Regeneration in Aqueous/Organic Two-Liquid Phase Systems. Top Catal 57, 385–391 (2014). https://doi.org/10.1007/s11244-013-0195-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0195-y