Abstract
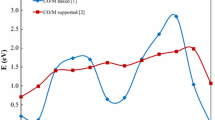
We present the reaction pathways and potential energy landscapes for photo-oxidation of methyl chloride involving oxygen species on the rutile TiO2(110) surface. Bridging oxygen atoms native to the TiO2(110) surface prove insufficient as H acceptors in the reaction. Rather oxygen atoms or hydroxyl groups bound at terminal Ti sites in the Ti troughs are required to facilitate the photo-oxidation, which can proceed all the way to formaldehyde. In the calculations, a photo-generated hole in the TiO2 valence band is assumed, while the corresponding photo-electron is omitted from the models. This prohibits the electron–hole recombination and changes the photo oxidation reaction into a fictitious electronic ground state problem, tractable by density functional theory.





Similar content being viewed by others
References
Fujishima A, Zhang X, Tryk DA (2008) Surf Sci Rep 63:515
Diebold U (2003) Surf Sci Rep 48:53
Linsebigler A, Lu G, Yates JT (1996) J Phys Chem 100:6631
Nadeem AM, Muir JMR, Connelly KA, Adamson BT, Metson BJ, Idriss H (2011) Phys Chem Chem Phys 13:7637
Shiraishi Y, Togawa Y, Tsukamoto D, Tanaka S, Hirai T (2012) ACS Catal 2:2475
Wang Z-T, Deskins NA, Henderson MA, Lyubinetsky I (2012) Phys Rev Lett 109:266103
Wendt S, Sprunger PT, Lira E, Madsen GKH, Li Z, Hansen J, Matthiesen J, Blekinge-Rasmussen A, Lægsgaard E, Hammer B, Besenbacher F (2008) Science 320:1755
Henderson MA (2005) J Phys Chem B 109:12062
Shen M, Henderson MA (2012) J Phys Chem C 116:18788
Phillips KR, Jensen SC, Baron M, Li S-C, Friend CM (2013) J Am Chem Soc 135:574
Lu G, Linsebigler A, Yates JT (1995) J Phys Chem 99:7626
Scheiber P, Riss A, Schmid M, Varga P, Diebold U (2010) Phys Rev Lett 105:216101
Petrik NG, Kimmel GA (2010) J Phys Chem Lett 1:1758
Lira E, Hansen JØ, Huo P, Bechstein R, Galliker P, Lægsgaard E, Hammer B, Wendt S, Besenbacher F (2010) Surf Sci 604:1945
Bikondoa O, Pang CL, Ithnin R, Muryn CA, Onishi H, Thornton G (2006) Nat Mater 5:189
Matthiesen J, Wendt S, Hansen JØ, Madsen GKH, Lira E, Galliker P, Vestergaard EK, Schaub R, Lægsgaard E, Hammer B, Besenbacher F (2009) ACS Nano 3:517
Yoshihara T, Katoh R, Furube A, Tamaki Y, Murai M, Hara K, Murata S, Arakawa H, Tachiya M (2004) J Phys Chem B 108:3817
Linsebigler AL, Lu G, Yates JT (1995) Chem Rev 95:735
Henderson MA, Lyubinetsky I (2013) Chem Rev 113:4428
Wanbayor R, Deák P, Frauenheim T, Ruangpornvisuti V (2011) J Chem Phys 134:104701
Di Valentin C, Fittipaldi D (2013) J Phys Chem Lett 4:1901
Chrétien S, Metiu H (2008) J Chem Phys 128:044714
Martinez U, Hammer B (2011) J Chem Phys 134:194703
Mortensen JJ, Hansen LB, Jacobsen KW (2005) Phys Rev B 71:035109
Enkovaara J, Rostgaard C, Mortensen JJ, Chen J, Dułak M, Ferrighi L, Gavnholt J, Glinsvad C, Haikola V, Hansen HA, Kristoffersen HH, Kuisma M, Larsen AH, Lehtovaara L, Ljungberg M, Lopez-Acevedo O, Moses PG, Ojanen J, Olsen T, Petzold V, Romero NA, Stausholm-Møller J, Strange M, Tritsaris GA, Vanin M, Walter M, Hammer B, Häkkinen H, Madsen GKH, Nieminen RM, Nørskov JK, Puska M, Rantala TT, Schiøtz J, Thygesen KS, Jacobsen KW (2010) J Phys Condens Matter 22:253202
Wellendorff J, Lundgaard KT, Møgelhøj A, Petzold V, Landis DD, Nørskov JK, Bligaard T, Jacobsen KW (2012) Phys Rev B 85:235149
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Henkelman G, Uberuaga BP, Jónsson H (2000) J Chem Phys 113:9901
Bahn SR, Jacobsen KW (2002) Comput Sci Eng 4:56
Micic OI, Zhang Y, Cromack KR, Trifunac AD, Thurnauer MC (1993) J Phys Chem 97:7277
Nakaoka Y, Nosaka Y (1997) J Photochem Photobiol A 110:299
Deskins NA, Dupuis M (2009) J Phys Chem C 113:346
Ji Y, Wang B, Luo Y (2012) J Phys Chem C 116:7863
Zawadzki P, Jacobsen KW, Rossmeisl J (2011) Chem Phys Lett 506:42
Di Valentin C, Selloni A (2011) J Phys Chem Lett 2:2223
Stausholm-Moller J, Kristoffersen HH, Hinnemann B, Madsen GKH, Hammer B (2010) J Chem Phys 133:144708
Iwaszuk A, Nolan M (2011) J Phys Condens Matter 23:334207
Ghuman KK, Singh CV (2013) J Phys Condens Matter 25:085501
Di Valentin C, Pacchioni G, Selloni A (2006) Phys Rev Lett 97:166803
Acknowledgments
We wish to congratulate J. K. Nørskov on the occasion of his 60th year anniversary, and one of us (BH) would like to express his gratitude for being introduced to the field of computational surface science and catalysis, for many fruitful collaborative projects and for steady support and inspiration. This work was supported by the Danish Research Councils, COST action CM1104, and Danish Center for Scientific Computing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kristoffersen, H.H., Martinez, U. & Hammer, B. Modeling Methyl Chloride Photo Oxidation by Oxygen Species on TiO2(110). Top Catal 57, 171–176 (2014). https://doi.org/10.1007/s11244-013-0173-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0173-4