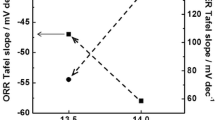
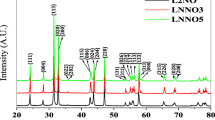
Abstract
Electrocatalytic oxygen reduction reaction (ORR) activities of the pyrochlore oxides Ln2Zr2O7−δ (LnZ) and Ln2Sn2O7−δ (LnS) (Ln = La, Pr, Nd, Sm) were examined in 0.1 M KOH solution at 70 °C. The onset potential (E on) of the oxygen reduction current and the efficiency (Eff 4) of 4-electron reduction of oxygen were evaluated by semi-steady state voltammetry with a rotating ring-disk electrode. In both LnZ and LnS series, the E on values were ~0.85 V versus reversible hydrogen electrode. A relation was found between the E on values and the lattice parameters; i.e. on the whole, the ORR activity became high with an increase in the lattice parameters. When the Ln ion was the same, the LnZ series exhibited higher ORR activities than the LnS series. The pyrochlore LaZ with the highest ORR activity showed a Eff 4 value higher than 85%. Moreover, Mn-incorporation to LaZ led to a mixed-oxide (1–xLaZ−xLaM) of LaZ and the perovskite LaMnO3 (LaM). However, the E on value apparently sifted to a more positive potential probably due to LaMnO3, and the magnitude of the cathodic ORR current increased with an increase in the mixing content up to x = 0.3. The mixed-oxide 0.7LaZ–0.3LaM exhibited the highest ORR activity (E on = ~0.90 V and Eff 4 > 95%), which was comparable to that of a conventional 20 mass% Pt/C catalyst.











Similar content being viewed by others
References
Vielstich W, Lamm A, Gasteiger H (eds) (2003) Handbook of fuel cells: fundamentals technology and applications (vols. 1–4). John Wiley & Sons, Chichester
Gelling PJ, Bouwmeester HJM (eds) (1997) The handbook of solid state electrochemistry. CRC Press, Baca Raton, Florida, pp 329–407
Haile SM, Boysen DA, Chisholm CRI, Merle RB (2001) Nature 410:910
Horowitz HS, Longo JM, Lewandowski JT (1978) US Patent 4,129,525 (1978)
Zen J-M, Manoharan R, Goodenough JB (1992) J Appl Electrochem 22:140
Yoshihara K, Saito Y, Saito M, Kuwano J, Shiroishi H (2007) Key Eng Mater 350:171
Saito Y, Yokota K, Yoshihara K, Saito M, Kuwano J, Shiroishi H (2007) Key Eng Mater 350:167
Kuwano J, Saito M (2008) Japan Patent 2008-123460
Saito M, Saito Y, Konishi T, Kawai H, Kuwano J, Shiroishi H, Uchimoto Y (in press) ECS transaction
Yamamoto T, Kanno R, Takeda Y, Yamamoto O, Kawamoto Y, Takano M (1994) J Solid State Chem 109:372
Yamamura H, Nishino H, Kakinuma K, Nomura K (2003) Solid State Ionics 158:359
Imaizumi S, Shimanoe K, Teraoka Y, Yamazoe N (2005) Electrochem Solid State Lett 8:A270
Miyazaki K, Sugimura N, Matsuoka K, Iriyama Y, Abe T, Matsuoka M, Ogumi Z (2008) J Power Sources 178:683
Miyazaki K, Iriyama Y, Abe T, Matsuoka M, Ogumi Z (2005) J Power Sources 150:27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konishi, T., Kawai, H., Saito, M. et al. Electrocatalytic Activity of the Pyrochlores Ln2M2O7−δ (Ln = Lanthanoids) for Oxygen Reduction Reaction. Top Catal 52, 896–902 (2009). https://doi.org/10.1007/s11244-009-9223-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-009-9223-3