Abstract
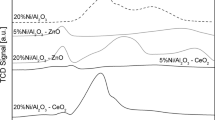
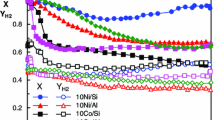
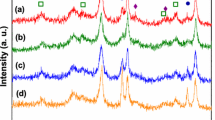
The ethanol steam reforming has been investigated over supported cobalt catalysts at atmospheric pressure. About 12% cobalt was supported on Al2O3, SiO2 and TiO2, and a commercial Ni/Al2O3 catalyst (G90B) was included for comparative purposes. The selectivity was found to depend strongly on the support, especially at low and medium temperatures. The initial activity of the cobalt catalysts correlated well with the metal dispersion. Acetaldehyde was an important C-containing product at low temperatures, whereas at high temperatures CO, CO2 and CH4 dominated the product spectrum. A significant production of ethene was observed, especially on the alumina-supported catalysts. The results are in agreement with a mechanism involving acetaldehyde as an intermediate in the steam reforming. At high temperatures (>550 °C) the conversion was complete and the product distribution approaches the equilibrium. The H2 yield approached 5 moles H2/mole ethanol converted, which is close to the maximum according to thermodynamic calculations. The alumina-supported catalysts (both Co and Ni) showed acceptable deactivation rates, but high carbon formation.






Similar content being viewed by others
References
Adamson KA (2004) Energy Policy 32:1231
Rostrup-Nielsen J (2001) PhysChem Chem Phys 3:283
Deluga GA, Salge JR, Schmidt LD, Verykios XE (2004) Science 303:993
Sheng P-Y, Yee A, Bowmaker GA, Idriss H (2002) J Catal 208:393
Garcia EY, Laborde MA (1991) Int J Hydrogen Energy 16:307
Aupretre F, Descorme C, Duprez D (2002) Catal Commun 3:263
Fatsikostas AN, Kondarides DI, Verykios XE (2002) Catal Today 75:145
Freni S, Cavallaro S, Mondello N, Spadaro L, Frusteri F (2002) J Power Sources 108:53
Freni S, Cavallaro S, Mondello N, Spadaro L, Frusteri F (2003) Catal Commun 4:259
Liguras DK, Goundani K, Verykios XE (2004) Int J Hydrogen Energy 29:419
Sekine Y, Asai S, Urasaki K, Matsukata M, Kikuchi E, Kado S., Haga F (2005) Chem Lett (Jpn) 34:658
Llorca J, Homs N, Ramirez de la Piscina P (2004) J Catal 227:556
Haga F, Nakajima T, Yamashita K, Mishima S (1998) React Kinet Catal Lett 63:253
Kaddouri A, Mazzocchia C (2004) Catal Commun 5:339
Storsæter S, Borg Ø, Blekkan EA, Holmen A (2005) J Catal 231:405
Goula MA, Kontou SK, Tsiakaras PE (2004) Appl Catal B: Environ 49:135
Haga F, Nakajima T, Miya H, Mishima S (1997) Catal Lett 48:223
Batista MS, Santos RKS, Assaf EM, Assaf JM, Ticianelli EA (2004) J Power Sources 134:27
Comas J, Marino F, Laborde M, Amadeo N (2004) Chem Eng J 98:61
Fatsikostas AN, Verykios XE (2004) J Catal 225:439
Abd El-Raady AA, Fouad NE, Mohamed MA, Halawy SA (2005) Monatsh Chem 133:1351
Cavallaro S (2000) Energy Fuels 14:1195
Rostrup-Nielsen J, Trimm DL (1977) J Catal 48:155
Acknowledgments
The Norwegian Research Council is acknowledged for financial support through the KOSK programme. O.A. Rokstad is thanked for assistance with thermodynamic calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bichon, P., Haugom, G., Venvik, H.J. et al. Steam Reforming of Ethanol Over Supported Co and Ni Catalysts. Top Catal 49, 38–45 (2008). https://doi.org/10.1007/s11244-008-9061-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-008-9061-8