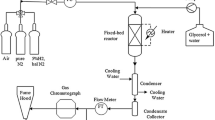
The kinetics of the glycerol oxidation using a carbon supported gold catalyst was studied experimentally in a batch reactor at oxygen pressures up to 10 bar and at temperatures from 25 to 100 °C. The influence of the mass transfer on the reaction was estimated and confirmed with theoretical calculations. A kinetic model has been proposed on the basis of a Langmuir-Hinshelwood mechanism for the experiments carried out in the kinetic regime and the kinetic parameters (reaction rate and adsorption constants as well as activation energies) were calculated.
Similar content being viewed by others
Abbreviations
- a p :
-
external area of particles per unit volume of reactor [m2/m3]
- C 1 :
-
glycerol concentration [mol L−1]
- C 2 :
-
dihydroxyacetone concentration [mol L−1]
- C 2al :
-
glyceraldehyde concentration [mol L−1]
- C 3 :
-
glyceric acid concentration [mol L−1]
- C 4 :
-
tartronic acid concentration [mol L−1]
- C 5 :
-
glycolic acid concentration [mol L−1]
- C 6 :
-
oxalic acid concentration [mol L−1]
- d :
-
impeller diameter [cm]
- d p :
-
average catalyst particle diameter [m]
- D :
-
molecular diffusion coefficient [m2/s]
- D eff :
-
effective diffusion coefficient [m2/s]
- e :
-
rate of flow energy supply per unit mass of liquid [m2/s3]
- E A :
-
activation energy [J/mol]
- Fr :
-
Froude number [−]
- H i :
-
Henrýs coefficient [kPa m3/mol]
- k 1 ... k6 :
-
reaction rate constants \({\hbox{[L}}^{{\rm{(n}}_{\rm{i}} + {\rm{ n}}_{{\rm{bi}}} - {\rm{1)}}} {\hbox{/(mol}}^{{\rm{n}}_{\rm{i}} + {\rm{ n}}_{{\rm{bi}}} - {\rm{1}}} {\hbox{ min g)]}}\)
- k 01 ... k 06 :
-
pre-exponential factors \({\hbox{[L}}^{{\rm{(n}}_{\rm{i}} + {\rm{ n}}_{{\rm{bi}}} - {\rm{1)}}} {\hbox{/(mol}}^{{\rm{n}}_{\rm{i}} + {\rm{ n}}_{{\rm{bi}}} - {\rm{1}}} {\hbox{ min g)]}}\)
- K 1 ... K 6 :
-
adsorption equilibrium constants [l/mol]
- k l :
-
gas–liquid mass transfer coefficient [cm/s]
- k l a :
-
volumetric gas–liquid mass transfer coefficient [s−1]
- k s :
-
liquid-solid mass transfer coefficient [cm/s]
- n 1 ... n 6 :
-
reaction order with respect to the educt of step 1 ... 6 [−]
- n b :
-
reaction order with respect to the base concentration [−]
- N :
-
speed of agitation [Hz]
- N P :
-
power number [−]
- P :
-
oxygen pressure [kPa]
- r 1 ... r 6 :
-
reaction rates of respective steps in figure 1 [mol/L min]
- Re :
-
Reynolds number [−]
- Sc :
-
Schmidt number [−]
- V :
-
reaction volume [m3]
- V s :
-
superficial gas velocity [cm/s]
- w :
-
catalyst amount [g]
- αl :
-
ratio chemical reaction/gas–liquid mass transfer [−]
- αs :
-
ratio chemical reaction/liquid–solid mass transfer [−]
- Φexp :
-
Thiele modulus [−]
- ε:
-
catalyst porosity [−]
- μ:
-
viscosity of the liquid [g/cm s]
- ν:
-
kinematic viscosity [cm2/s]
- σ:
-
surface tension [g/s2]
- ρ:
-
density of the liquid [g/cm3]
- ψ:
-
shape factor [−]
- τ:
-
catalyst tortuosity [−]
- ω:
-
ratio catalyst weight to reactor volume [kg/m3]
References
F. Porta L. Prati (2004) J. Catal. 224 397 Occurrence Handle10.1016/j.jcat.2004.03.009 Occurrence Handle1:CAS:528:DC%2BD2cXjvVGis7s%3D
S. Carrettin P. McMorn P. Johnston K. Griffin C.J. Kiely G.J. Hutchings (2003) Phys. Chem. Chem. Phys. 5 1329 Occurrence Handle10.1039/b212047j Occurrence Handle1:CAS:528:DC%2BD3sXhsFKrt7w%3D
H. Kimura and K. Den, JP 04-210665 (1992)
N. Dimitratos A. Villa L. Bianchi L. Prati M. Makkee (2006) Appl. Catal. A 311 185 Occurrence Handle10.1016/j.apcata.2006.06.026 Occurrence Handle1:CAS:528:DC%2BD28XotlWnu7c%3D
S. Claude (1999) Fett/Lipid 101 101 Occurrence Handle10.1002/(SICI)1521-4133(199903)101:3<101::AID-LIPI101>3.0.CO;2-4 Occurrence Handle1:CAS:528:DyaK1MXitFOqsLY%3D
S. Demirel-Gülen M. Lucas P. Claus (2005) Catal. Today 102–103 166 Occurrence Handle10.1016/j.cattod.2005.02.033
S. Demirel K. Lehnert M. Lucas P. Claus (2007) Appl. Catal. B Environ. 70 637 Occurrence Handle10.1016/j.apcatb.2005.11.036 Occurrence Handle1:CAS:528:DC%2BD2sXivVCl
P.L. Mills R.V. Chaudhari (1997) Catal. Today 37 367 Occurrence Handle10.1016/S0920-5861(97)00028-X Occurrence Handle1:CAS:528:DyaK2sXmt1Sjs70%3D
H. Yagi F. Yoshida (1975) Ind. Eng. Chem. Proc. Des. Dev. 14 4 Occurrence Handle10.1021/i260056a024
Y. Sano N. Yamaguchi T. Adachi (1974) J. Chem. Eng. Japan 7 4
E. Rischbieter A. Schumpe V. Wunder (1996) J. Chem. Eng. Data 41 809 Occurrence Handle10.1021/je960039c Occurrence Handle1:CAS:528:DyaK28Xjs1Ogt7k%3D
H. Haario, Modest 6.0 user’s guide, Profmath Oy, Helsinki, 2001
U. Hoffmann and M. Hoffmann, Einführung in die Optimierung mit Anwendungsbeispiele aus dem Chemie-Ingenieur-Wesen (Verlag Chemie GmbH, Weinheim, 1971)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demirel, S., Lucas, M., Wärnå, J. et al. Reaction kinetics and modelling of the gold catalysed glycerol oxidation. Top Catal 44, 299–305 (2007). https://doi.org/10.1007/s11244-007-0303-y
Issue Date:
DOI: https://doi.org/10.1007/s11244-007-0303-y