Abstract
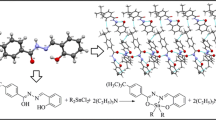
Five new organotin(IV) complexes, Me2SnL2 (1), n–Bu2SnL2 (2), t–Bu2SnL2 (3), Ph2SnL2 (4), and Ph3SnL (5), have been designed and synthesized by the reactions of the deprotonated 1-adamantanethiol ligand (L = C10H15S) with the corresponding R2SnCl2 (R = Me, n–Bu, t–Bu, Ph) and Ph3SnCl. The complexes were characterized by elemental analysis, FT-IR, NMR spectroscopy, and X-ray crystallography. Meanwhile, optimized geometrical parameters, harmonic vibrational frequencies, and frontier molecular orbitals were calculated. The in vitro cytotoxicities of the complexes were evaluated with HeLa and HepG-2. Furthermore, the antifungal activity of the newly synthesized complexes has been evaluated, and the SEM and TEM images were prepared from Alternaria kikuchiana Tanaka to analyze the macroscopic action of the drug on the fungus. As a result, complex 5 has good antifungal activity and cytotoxicity.




Similar content being viewed by others
Availability of data and materials
Data available within the article or its supplementary materials.
References
Lawson JS, Glenn WK (2020) Infect Agent Cancer 15:41
Lewis EB (2004) In: Lipshitz HD (ed) Ionizing radiation, cancer induction and radioactive fallout. Springer
Armstrong BK (2004) How sun exposure causes skin cancer: an epidemiological perspective. Springer, Netherlands
Koual M, Tomkiewicz C, Cano-Sancho G, Antignac JP, Bats AS, Coumoul X (2020) Environ Health Perspect 19:117
Szorcsik A, Nagy L, Gajda-Schrantz K, Pellerito L, Nagy E, Edelmann FT (2002) J Radioanal Nucl Chem 252:523–530
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) CA Cancer J Clin 71:209–249
Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I (2022) J Hepatol 77:1598–1606
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le Chevalier T (2008) J Clin Oncol 26:3552–3559
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Am J Med Sci 334:115–124
Dasari S, Tchounwou PB (2014) Eur J Pharmacol 740:364–378
Swaminathan S, Haribabu J, Balakrishnan N, Vasanthakumar P, Karvembu R (2022) Coord Chem Rev 459:214403
Haribabu J, Tamura Y, Yokoi K, Balachandran C, Umezawa M, Tsuchiya K, Yamada Y, Karvembu R, Aoki S (2021) Eur J Inorg Chem 2021:1796–1814
Haribabu J, Srividya S, Mahendiran D, Gayathri D, Venkatramu V, Bhuvanesh N, Karvembu R (2020) Inorg Chem 59:17109–17122
Al-Resayes SI, Shakir M, Shahid N, Azam M, Khan AU (2016) Arab J Chem 9:335–343
Azam M, Warad I, Al-Resayes S, Shakir M, Ullah MF, Ahmad A, Sarkar FH (2012) Inorg Chem Commun 20:252–258
Mohapatra RK, El-ajaily MM, Alassbaly FS, Sarangi AK, Das D, Maihub AA, Ben-Gweirif SF, Mahal A, Suleiman M, Perekhoda L, Azam M, Al-Noor TH (2021) Chem Pap 75:1005–1019
Amir MK, Khan S, Ziaur R, Shah A, Butler IS (2014) Inorg Chim Acta 423:14–25
Zhang Q, Zhang M, Wang H, Tian X, Ma W, Luo L, Wu J, Zhou H, Li S, Tian Y (2019) J Inorg Biochem 192:1–6
Ruan BF, Tian YP, Zhou HP, Wu JY, Hu RT, Zhu CH, Yang JX, Zhu HL (2011) Inorg Chim Acta 365:302–308
Dawara L, Singh RV (2011) Appl Organomet Chem 25:643–652
Azizur R, Hussain M, Ziaur R, Rauf A, Nasim FUH, Tahir AA, Ali S (2010) J Organomet Chem 695:1526–1532
Jain M, Maanju S, Singh RV (2004) Appl Organomet Chem 18:471–479
Mariam S, Hussain S, Ali S, Shahzadi S, Ramzan S, Shahid M (2018) Iran J Sci Technol Trans A Sci 42:1277–1284
Rocha CS, de Morais BP, Rodrigues BL, Donnici CL, de Lima GM, Ardisson JD, Takahashi JA, Bitzer RS (2017) Appl Organomet Chem 31:e3645
Banti CN, Hadjikakou SK, Sismanoglu T, Hadjiliadis N (2019) J Inorg Biochem 194:114–152
Hadjikakou SK, Hadjiliadis N (2009) Coord Chem Rev 253:235–249
Annuar SNS, Kamaludin NF, Awang N, Chan KM (2021) Front Chem 9:657599
Attanzio A, D’Agostino S, Busa R, Frazzitta A, Rubino S, Girasolo MA, Sabatino P, Tesoriere L (2020) Molecules 25:e3645
Reiser J, McGregor E, Jones J, Enick R, Holder G (1996) Fluid Phase Equilib 117:160–167
Liu J, Obando D, Liao V, Lifa T, Codd R (2011) Eur J Med Chem 46:1949–1963
Wanka L, Iqbal K, Schreiner PR (2013) Chem Rev 113:3516–3604
Chew CF, Guy A, Biggin PC (2008) Biophys J 95:5627–5636
Yang JS, Han Z, Dong XY, Luo P, Mo HL, Zang SQ (2020) Angew Chem Int Ed Engl 59:11898–11902
Takaoka A, Mankad NP, Peters JC (2011) J Am Chem Soc 133:8440–8443
Agapie T, Odom AL, Cummins CC (2000) Inorg Chem 39:174–179
Delcaillau T, Bismuto A, Lian Z, Morandi B (2020) Angew Chem Int Ed Engl 59:2110–2114
Mikołajczyk M, Łyżwa P, Drabowicz J, Wieczorek M, Bujacz G (1989) Angew Chem Int Ed Engl 28:97–98
Feng W, Ye K, Jiang Y, Hou R (2019) Chem Res Chin Univ 35:556–559
Sainorudin MH, Sidek NM, Ismail N, Rozaini MZH, Harun NA, Sabiqah Tuan Anuar TN, Abd Rahman Azmi AA, Yusoff F (2015) J Chem Sci 2:5692
Casas JS, Castineiras A, Haiduc I, Sánchez A, Semeniuc RF, Sordo J (2007) Synth React Inorg Met-Org Chem 31:725–736
Lockhart TP, Manders WF (2002) Inorg Chem 25:892–895
Armstrong D, Taullaj F, Singh K, Mirabi B, Lough AJ, Fekl U (2017) Dalton Trans 46:6212–6217
Holeček J, Nádvorník M, Handlíř K, Lyčka A (1986) J Organomet Chem 315:299–308
Sair U, Thakur A (2022) Mater 50:1862–1866
Varga RA, Schuermann M, Silvestru C (2001) J Organomet Chem 623:161–167
Neese F (2022) Wiley Interdiscip Rev Comput Mol 12:e1606
Lu T, Chen F (2012) J Comput Chem 33:580–592
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) J Cheminform 4:17
Singh HL, Singh JB, Bhanuka S (2015) Res Chem Intermed 42:997–1015
Fukui K (1982) Science 218:747–754
Javed F, Sirajuddin M, Ali S, Khalid N, Tahir MN, Shah NA, Rasheed Z, Khan MR (2016) Polyhedron 104:80–90
Singh HL, Singh JB, Bhanuka S (2016) Res Chem Intermed 42:997–1015
Haribabu J, Garisetti V, Malekshah RE, Srividya S, Gayathri D, Bhuvanesh N, Mangalaraja RV, Echeverria C, Karvembu R (2022) J Mol Struct 1250:131782
Haribabu J, Alajrawy OI, Jeyalakshmi K, Balachandran C, Krishnan DA, Bhuvanesh N, Aoki S, Natarajan K, Karvembu R (2021) SAA 246:118963
Haribabu J, Balakrishnan N, Swaminathan S, Peter J, Gayathri D, Echeverria C, Bhuvanesh N, Karvembu R (2021) Inorg Chem Commun 134:109029
Wang S, Li QL, Zhang RF, Du JY, Li YX, Ma CL (2019) Polyhedron 158:15–24
Nath M, Vats M, Roy P (2015) J Photochem Photobiol B: Biol 148:88–100
El-Barasi NM, Miloud MM, El-ajaily MM, Mohapatra RK, Sarangi AK, Das D, Mahal A, Parhi PK, Pintilie L, Barik SR, Bitu MNA, Kudrat-E-Zahan M, Tabassum Z, Al-Resayes SI, Azam M (2020) J Saudi Chem Soc 24:492–503
Azam M, Al-Resayes SI, Wabaidur SM, Altaf M, Chaurasia B, Alam M, Shukla SN, Gaur P, Albaqami NTM, Islam MS, Park S (2018) Molecules 23:813
Guo Q, Zhang RF, Hua XW, Li QL, Du XM, Ru J, Ma CL (2022) New J Chem 46:4314–4324
Cui ZN, Li YS, Hu DK, Tian H, Jiang JZ, Wang Y, Yan XJ (2016) Sci Rep 6:20204
Li D, Calderone R (2017) Virulence 8:159–168
SAINT Software Users Guide, version 7.0. (1999) Bruker analytical X-ray systems, Madison
Sheldrick GM (2000) SADABS, version 2.03. Bruker analytical X-ray systems, Madison
Sheldrick GM (2003) SHELXTL, Version 6.14. Bruker AXS, Inc., Madison
Sheldrick GM (2015) Acta Crystallogr C Struct Chem 71:3–8
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Crystallogr 42:339–341
Neese F (2011) Wiley Interdiscip Rev Comput Mol Sci 1:73–78
Weigend F (2006) Phys Chem Chem Phys 8:1057–1065
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Grimme S, Ehrlich S, Goerigk L (2011) J Comput Chem 32:1456–1465
Cao X, Dolg M (2001) Chem Phys 115:7348–7355
Chandrasekar S, Balachandran V, Evans HS, Latha A (2015) SAA 143:136–146
Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z, Schwartz B (2002) J Nutr 132:3754–3759
Funding
This research was funded by National Natural Science Foundation of China (21371087) and Natural Science Foundation of Shandong Province (ZR2020MB019).
Author information
Authors and Affiliations
Contributions
ZM synthesized the complexes, conducted most of the measurements, and wrote the original draft. HS collected the crystal structures. RZ and CM designed the study and supervised the project. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, Z., Zhang, R., Shi, H. et al. New organotin(IV) complexes derived from 1-adamantanethiol: synthesis, crystal structure, DFT calculation, and in vitro antifungal activity and cytotoxicity. Transit Met Chem 48, 113–124 (2023). https://doi.org/10.1007/s11243-023-00528-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-023-00528-9