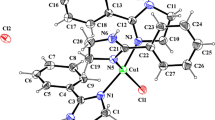
Abstract
Bis(benzotriazol-1-yl)phenylmethane CHPh(btz)2 and tris(benzotriazol-1-yl)methane CH(btz)3 were used as N-donor ligands to prepare luminescent heteroleptic copper(I) complexes. [Cu{CHPh(btz)2}(PPh3)2][BF4] and [Cu{CHPh(btz)2}(DPEphos)][BF4] (DPEphos = bis[(2-diphenylphosphino)phenyl] ether) were obtained from the corresponding borohydride complexes [Cu(BH4)(PPh3)2] and [Cu(BH4)(DPEphos)] and tetrafluoroboric acid. [Cu{CH(btz)3}(PPh3)][BF4] and [Cu{CH(btz)3}(PiPr3)][BF4] were prepared from the acetonitrile complex [Cu(NCCH3)4][BF4]. The complexes exhibited bright yellow or orange emissions upon excitation with near-UV and violet light. The photoluminescent properties were attributed to metal-to-ligand charge transfer transitions on the basis of experimental data and DFT calculations.
Graphical abstract








Similar content being viewed by others
References
Wenger OS (2018) Photoactive complexes with earth-abundant metals. J Am Chem Soc 140:13522–13533. https://doi.org/10.1021/jacs.8b08822
Zhang QC, Xiao H, Zhang X, Xu LJ, Chen ZN (2019) Luminescent oligonuclear metal complexes and the use in organic light-emitting diodes. Coord Chem Rev 378:121–133. https://doi.org/10.1016/j.ccr.2018.01.017
Costa RD, Ortí E, Bolink HJ, Monti F, Accorsi G, Armaroli N (2012) Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew Chem Int Ed 51:8178–8211. https://doi.org/10.1002/anie.201201471
Bizzarri C, Spuling E, Knoll DM, Volz D, Bräse S (2018) Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord Chem Rev 373:49–82. https://doi.org/10.1016/j.ccr.2017.09.011
Cariati E, Lucenti E, Botta C, Giovanella U, Marinotto D, Righetto S (2016) Cu(I) hybrid inorganic–organic materials with intriguing stimuli responsive and optoelectronic properties. Coord Chem Rev 306:566–614. https://doi.org/10.1016/j.ccr.2015.03.004
Armaroli N, Accorsi G, Cardinali F, Listorti A (2007) Photochemistry and photophysics of coordination compounds: copper. Top Curr Chem 280:69–115. https://doi.org/10.1007/128_2007_128
Tao Y, Yuan K, Chen T, Xu P, Li H, Chen R, Zheng C, Zhang L, Huang W (2014) Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv Mater 26:7931–7958. https://doi.org/10.1002/adma.201402532
Yersin H, Rausch AF, Czerwieniec R, Hofbeck T, Fischer T (2011) The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord Chem Rev 255:2622–2652. https://doi.org/10.1016/j.ccr.2011.01.042
Yersin H (2018) Highly efficient OLEDs: materials based on thermally activated delayed fluorescence. Wiley-VCH, Weinheim
McMillin DR, Buckner MT, Ahn BT (1977) A light-induced redox reaction of bis(2,9-dimethyl-1,10-phenanthroline)copper(I). Inorg Chem 16:943–945. https://doi.org/10.1021/ic50170a046
Buckner MT, McMillin DR (1978) Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J Chem Soc Chem Commun, pp 759–761. https://doi.org/10.1039/C39780000759
Blaskie MW, McMillin DR (1980) Photostudies of copper(I) systems. 6. Room-temperature emission and quenching studies of bis(2,9-dimethyl-1,10-phenanthroline)copper(I). Inorg Chem 19:3519–3522. https://doi.org/10.1021/ic50213a062
Scaltrito DV, Thompson DW, O’Callaghan JA, Meyer GJ (2000) MLCT excited states of cuprous bis-phenanthroline coordination compounds. Coord Chem Rev 208:243–266. https://doi.org/10.1016/S0010-8545(00)00309-X
Lavie-Cambot A, Cantuela M, Leydet Y, Jonusauskas G, Bassani DM, McClenaghan ND (2008) Improving the photophysical properties of copper(I) bis(phenanthroline) complexes. Coord Chem Rev 252:2572–2584. https://doi.org/10.1016/j.ccr.2008.03.013
Liu Y, You SC, Ho CL, Wong WY (2018) Recent advances in copper complexes for electrical/light energy conversion. Coord Chem Rev 375:514–557. https://doi.org/10.1016/j.ccr.2018.05.010
Si Z, Li J, Li B, Liu S, Li W (2009) High light electroluminescence of novel Cu(I) complexes. J Lumin 129:181–186. https://doi.org/10.1016/j.jlumin.2008.09.014
Min J, Zhang Q, Sun W, Cheng Y, Wang L (2011) Neutral copper(I) phosphorescent complexes from their ionic counterparts with 2-(2′-quinolyl)benzimidazole and phosphine mixed ligands. Dalton Trans 40:686–693. https://doi.org/10.1039/C0DT01031F
Bergmann L, Braun C, Nieger M, Bräse S (2018) The coordination- and photochemistry of copper(I) complexes: variation of N^N ligands from imidazole to tetrazole. Dalton Trans 47:608–621. https://doi.org/10.1039/C7DT03682E
Gérardy R, Monbaliu JCM (2016) Preparation, reactivity, and synthetic utility of simple benzotriazole derivatives. The chemistry of benzotriazole derivatives. Topics in Heterocyclic Chemistry 43:1–66. https://doi.org/10.1007/7081_2015_179
Katritzky AR, Rogovoy BV (2003) Benzotriazole: an ideal synthetic auxiliary. Chem Eur J 9:4586–4593. https://doi.org/10.1002/chem.200304990
Loukopoulos E, Kostakis GE (2019) Recent advances in the coordination chemistry of benzotriazole-based ligands. Coord Chem Rev 395:193–229. https://doi.org/10.1016/j.ccr.2019.06.003
Richardson C, Steel PJ (2003) Benzotriazole as a structural component in chelating and bridging heterocyclic ligands; ruthenium, palladium, copper and silver complexes. Dalton Trans, pp 992–1000. https://doi.org/10.1039/b206990c
Peresypkina EV, Lider EV, Smolentsev AI, Sanchiz J, Gil-Hernández B, Potapov AS, Khlebnikov AI, Kryuchkova NA, Lavrenova LG (2012) Bis(benzotriazol-1-yl)methane as a linker in the assembly of new copper(II) coordination polymers: Synthesis, structure and investigations. Polyhedron 48:253–263. https://doi.org/10.1016/j.poly.2012.08.072
Belousov YA, Goncharenko VE, Bondarenko GN, Ganina OG, Bezzubov SI, Taidakov IV (2020) Linear metal-organic frameworks based on Bis(1-Benzotriazolyl)methane and zinc and copper nitrates. Russ J Coord Chem 46:805–811. https://doi.org/10.1134/S1070328420080023
Lobbia GG, Pellei M, Pettinari C, Santini C, Somers N, White AH (2002) Poly(1,2,3-benzotriazolyl)borate complexes with copper(I) and tri-organophosphane: an unprecedented κ1-coordination of [H2B(btz)2] (btz=1,2,3-benzotriazolyl) in the X-ray crystal structure of [Cu(PBn3)2{(btz)BH2(btz)}]. Inorg Chim Acta 333:100–108. https://doi.org/10.1016/S0020-1693(02)00774-0
Avila L, Elguero J, Juliá S, del Mazo JM (1983) N-Polyazolymethanes. IV. Reaction of Benzotriazole with Methylene Chloride and Chloroform under Phase Transfer Conditions. Heterocycles 20:1787–1792. https://doi.org/10.3987/R-1983-09-1787
Elguero J, Claramunt RM, Garcerán R, Julià S, Aliva L, del Mazo JM (1987) 13C NMR study of polyphenyl-, poly-N-azolyl-and poly-N-benzazoyl-methanes. Magn Reson Chem 25:260–268. https://doi.org/10.1002/mrc.1260250317
Katritzky AR, Xie L (1996) para-Formylation of nitroarenes via vicarious nucleophilic substitution of hydrogen with tris(benzotriazol-1-yl)methane. Tetrahedron Lett 37:347–350. https://doi.org/10.1016/0040-4039(95)02169-8
Katritsky AR, Wu H, Xie L (1997) Novel tele nucleophilic aromatic substitutions in α-(benzotriazol-1-yl)alkyl aryl ketones. Tetrahedron Lett 38:903–906. https://doi.org/10.1016/S0040-4039(96)02455-0
Androsov DA, Neckers DC (2007) Photochemical study of Tris(benzotriazol-1-yl)methane. J Org Chem 72:1148–1152. https://doi.org/10.1021/jo061851d
Bortoluzzi M, Castro J, Enrichi F, Vomiero A, Busato M, Huang W (2018) Green-emitting manganese (II) complexes with phosphoramide and phenylphosphonic diamide ligands. Inorg Chem Comm 92:145–150. https://doi.org/10.1016/j.inoche.2018.04.023
Bortoluzzi M, Castro J, Trave E, Dallan D, Favaretto S (2018) Orange-emitting manganese(II) complexes with chelating phosphine oxides. Inorg Chem Commun 90:105–107. https://doi.org/10.1016/j.inoche.2018.02.018
Bortoluzzi M, Castro J, Girotto M, Enrichi F, Vomiero A (2019) Luminescent copper(I) coordination polymer with 1-methyl-1H-benzotriazole, iodide and acetonitrile as ligands. Inorg Chem Comm 102:141–146. https://doi.org/10.1016/j.inoche.2019.02.016
Bortoluzzi M, Castro J (2019) Dibromomanganese(II) complexes with hexamethylphosphoramide and phenylphosphonic bis(diamide) ligands. J Coord Chem 72:309–327. https://doi.org/10.1080/00958972.2018.1560430
Bortoluzzi M, Castro J, Gobbo A, Ferraro V, Pietrobon L, Antoniutti (2020) Tetrahedral photoluminescent manganese(II) halide complexes with 1,3-dimethyl-2-phenyl-1,3-diazaphospholidine-2-oxide as a ligand. New J Chem 44:571–579. https://doi.org/10.1039/c9nj05083c
Bortoluzzi M, Castro J, Gobbo A, Ferraro V, Pietrobon L (2020) Light harvesting indolyl-substituted phosphoramide ligand for the enhancement of Mn(II) luminescence. Dalton Trans 49:7525–7534. https://doi.org/10.1039/d0dt01659d
Bortoluzzi M, Ferraro V, Castro J (2021) Synthesis and photoluminescence of manganese(II) naphtylphosphonic diamide complexes. Dalton Trans 50:3132–3136. https://doi.org/10.1039/D1DT00123J
Ferraro V, Bortoluzzi M, Castro J (2019) Synthesis of Bis(benzotriazol-1-yl)methane derivatives by Cobalt-catalyzed formation of C-C bonds. Proceedings 41:29. https://doi.org/10.3390/ecsoc-23-06469.
Ferraro V, Bortoluzzi M, Castro J, Vomiero A, You S (2020) Luminescent Cu(I) complex with bis(indazol-1-yl)phenylmethane as chelating ligand. Inorg Chem Commun 116:107894. https://doi.org/10.1016/j.inoche.2020.107894
Armarego WLF, Perrin DD (1996) Purification of laboratory chemicals, 4th edn. Butterworth-Heinemann, Oxford
Keller RN, Wycoff HD, Marchi LE (1946) Copper(I) chloride. Inorg Synth 2:1–4. https://doi.org/10.1002/9780470132333.ch1
Kubas GJ, Monzyk B, Crumblis AL (1990) Tetrakis(Acetonitrile)Copper(1+) Hexafluorophosphate(1-). Inorg Synth 28:68–70. https://doi.org/10.1002/9780470132593.ch15
Bianchini C, Ghilardi CA, Meli A, Midollini S, Orlandini A (1985) Reactivity of copper(I) tetrahydroborates toward carbon dioxide and carbonyl sulfide. Structure of (triphos)Cu(η1-O2CH). Inorg Chem 24:924–931. https://doi.org/10.1021/ic00200a025
Katritzky AR, Yang Z, Lam JN (1990) Tris(benzotriazol-1-yl)methane: A -CO2H synthon for the preparation of carboxylic acids. Synthesis 8:666–669. https://doi.org/10.1055/s-1990-26975
Bruker (2015) APEX3, SMART, SAINT. Bruker AXS Inc., Madison, Wisconsin, USA
McArdle P (2017) Oscail, a program package for small-molecule single-crystal crystallography with crystal morphology prediction and molecular modelling. J Appl Cryst 50:320–326. https://doi.org/10.1107/S1600576716018446
Sheldrick GM (2015) SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3–8. https://doi.org/10.1107/S2053229614024218
Spek AL (2009) Structure validation in chemical crystallography. Acta Crystallogr D 65:148–155. https://doi.org/10.1107/S090744490804362X
Grabowski SJ (2011) What is the covalency of hydrogen bonding? Chem Rev 111:2597–2625. https://doi.org/10.1021/cr800346f
Grabowski SJ (2016) Analysis of hydrogen bonds in crystals. Crystals 6:59 https://doi.org/10.3390/cryst6050059
Taylor R (2014) Which intermolecular interactions have a significant influence on crystal packing? CrystEngComm 16:6852–6865. https://doi.org/10.1039/C4CE00452C
Taylor R (2016) It isn’t, it is: the C-H···X (X = O, N, F, Cl) interaction really is significant in crystal packing. Cryst Growth Des 16:4165–4168. https://doi.org/10.1021/acs.cgd.6b00736
Aakeröy CB, Champness NR, Janiak C (2010) Recent advances in crystal engineering. CrystEngComm 12:22–43. https://doi.org/10.1039/B919819A
Gerber IC, Ángyán JC (2005) Hybrid functional with separated range. Chem Phys Lett 415:100–105. https://doi.org/10.1016/j.cplett.2005.08.060
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620. https://doi.org/10.1039/B810189B
Minenkov Y, Singstad Å, Occhipinti G, Jensen VR (2012) The accuracy of DFT-optimized geometries of functional transition metal compounds: a validation study of catalysts for olefin metathesis and other reactions in the homogeneous phase. Dalton Trans 41:5526–5541. https://doi.org/10.1039/C2DT12232D
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/B508541A
Ullrich CA (2012) Time-dependent density functional theory. Oxford University Press, Oxford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam J, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT.
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge structural database. Acta Crystallogr B72:171–179. https://doi.org/10.1107/S2052520616003954
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J Appl Cryst 41:466–470. https://doi.org/10.1107/S0021889807067908
Lu D, Tang H (2015) Theoretical survey of the ligand tunability of poly(azolyl)borates. Phys Chem Chem Phys 17:17027–17033. https://doi.org/10.1039/C5CP02094H
Kerscher T, Pust P, Betz R, Klüfers P, Mayer P (2009) Trispyrazol-1-ylmethane. Acta Crystallogr E 65:o108. https://doi.org/10.1107/S1600536808041767
Müller C, Koch A, Görls H, Krieck S, Westerhausen M (2015) Tris(pyrazolyl)methanides of the Alkaline Earth Metals: Influence of the Substitution Pattern on Stability and Degradation. Inorg Chem 54:635–645. https://doi.org/10.1021/ic5025907
Claramunt RM, López C, Jaime C, Virgili A, Marco C, Elguero J (1995) The conformation of Trispyrazolylmethanes: an experimental and theoretical study. Heterocycles 40:175–186. https://doi.org/10.3987/COM-94-S6
Ochando LE, Rius J, Louër D, Claramunt RM, López C, Elguero J, Amigó JM (1997) Phase transitions in Tris(3,5-dimethylpyrazol-1-yl)methane. The structure of the high-temperature phase from X-ray powder diffraction. Acta Crystallogr B 53:939–944. https://doi.org/10.1107/S0108768197007830
Molčanov K, Milašinović V, Kojić-Prodić B (2019) Contribution of different crystal packing forces in π-stacking: from noncovalent to covalent multicentric bonding. Cryst Growth Des 19:5967–5980. https://doi.org/10.1021/acs.cgd.9b00540
Mooibroek TJ, Gamez P (2012) How directional are D-H⋯phenyl interactions in the solid state (D = C, N, O)? CrystEngComm 14:8462–8467. https://doi.org/10.1039/C2CE26205C
Cabrera AR, Gonzalez IA, Cortés-Arriagada D, Natali M, Berke H, Daniliuc CG, Camarada MB, Toro-Labbé A, Rojasa RS, Salasa CO (2016) Synthesis of new phosphorescent imidoyl-indazol and phosphine mixed ligand Cu(I) complexes——structural characterization and photophysical properties. RCS Adv 6:5141–5153. https://doi.org/10.1039/C5RA20450J
Kubiček K, Veedu ST, Storozhuk D, Kia R, Techert S (2017) Geometric and electronic properties in a series of phosphorescent heteroleptic Cu(I) complexes: Crystallographic and computational studies. Polyhedron 124:166–176. https://doi.org/10.1016/j.poly.2016.12.035
Bizzarri C, Fléchon C, Fenwick O, Cacialli F, Polo F, Gálvez-López MD, Yang CH, Scintilla S, Sun Y, Fröhlich R, De Cola L (2016) Luminescent neutral Cu(I) complexes: synthesis, characterization and application in solution-processed OLED. ECS J Solid State Sci Technol 5:R83–R90. https://doi.org/10.1149/2.0021606jss
Kitai A (2008) Luminescent materials and applications. Wiley, Chichester
Ghassemlooy Z, Alves LN, Zvánovec S, Khalighi MA (2017) Visible light communications: theory and applications. CRC Press, Boca Raton
Acknowledgements
CACTI (University of Vigo) is gratefully acknowledged for X-ray data collection. We acknowledge Università Ca’ Foscari Venezia for financial support (Bando Spin 2018, D. R. 1065/2018 prot. 67416) and CINECA (COLUMN project 2020) for the availability of computing resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferraro, V., Castro, J., Agostinis, L. et al. Luminescent heteroleptic copper(I) complexes with polydentate benzotriazolyl-based ligands. Transit Met Chem 46, 391–402 (2021). https://doi.org/10.1007/s11243-021-00458-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-021-00458-4