Abstract
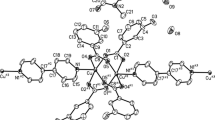
A Cu(II) coordination polymer, [Cu2(pztrz)2(μ-CH3COO)(CH3COO)]·3H2O (1), derived from mono-substituted 1,2,4-triazole derivative 3-(pyrazinyl)-1,2,4-triazole (Hpztrz) is isolated and structurally characterized. X-ray structural analysis shows that Hpztrz ligands and acetate anions demonstrate different coordination modes in complex 1. The two Cu(II) ions are bridged by the 1,2,4-triazole ring of Hpztrz to form a dinuclear unit, which is further linked by two bridging acetate anions to form a tetranuclear Cu4 unit. Interestingly, each Cu4 unit is connected to another four Cu4 units through the outer pyrazinyl of Hpztrz, resulting in a two-dimensional (2D) layer structure. Consecutive layers are further packed into three-dimensional (3D) structures through interlayer hydrogen-bonding interactions. Furthermore, variable-temperature magnetic susceptibility studies reveal that the antiferromagnetic interactions are mediated through 1,2,4-triazole-N1,N2 bridges and acetate bridges with coupling constants of J1 = − 69.98 cm−1 and J2 = − 2.15 cm−1.
Graphic abstract
Synergistic co-coordination of 1,2,4-triazole and acetate ions with Cu(II) ions led to a dinuclear unit, then to a Cu4 unit and finally to a 2D layer. Consecutive layers are further packed into a 3D framework through interlayer hydrogen-bonding interactions. Furthermore, variable-temperature magnetic susceptibility studies indicated the existence of antiferromagnetic coupling between the Cu(II) ions.









Similar content being viewed by others
References
Zaworotko MJ (2010) J Am Chem Soc 132:7821–7821
Caskey SR, Matzger AJ (2008) Inorg Chem 47:7942–7944
Chelebaeva E, Larionova J, Guari Y, Ferreira RAS, Carlos LD, Paz FAA, Trifonov A, Guérin C (2009) Inorg Chem 48:5983–5995
Srivastava S, Gupta BK, Gupta R (2017) Cryst Growth Des 17:3907–3916
Wang Y, Bao S, Li R, Zhao G, Wang Z, Zhao Z, Chen Q (2015) ACS Appl Mater Inter 7:2088–2096
Zhao XQ, Zhao B, Ma Y, Shi W, Cheng P, Jiang ZH, Liao DZ, Yan SP (2007) Inorg Chem 46:5832–5834
Shi WJ, Du LY, Yang HY, Zhang K, Hou L, Wang YY (2017) Inorg Chem 56:10090–10098
Zhai QG, Bai N, Li S, Bu X, Feng P (2015) Inorg Chem 54:9862–9868
Lin JB, Zhang JP, Zhang WX, Xue W, Xue DX, Chen XM (2009) Inorg Chem 48:6652–6660
Xu Z, Li H, Li A, Meng W, Hou H, Fan Y (2013) Inorg Chem Commun 36:126–129
Liu H, Gao G, Liu J, Bao F, Wei Y, Wang H (2019) CrystEngComm 21:2576–2584
Liu HY, Wang K, Sun Y, Wang R, Wang HY (2020) Inorg Chem 59:9452–9460
Liu HY, Liu J, Gao GM, Wang HY (2018) Inorg Chem 57:10401–10409
Liu H, Meng F, Lu Z, Bai J (2016) CrystEngComm 18:9003–9006
Liu H, Wang Q, Zhang M, Jiang J (2015) CrystEngComm 17:4793–4798
Liu HY, Chen LF, Wang HY, Wan Y, Wu H (2016) RSC Adv 6:94833–94839
Liu HY, Gao GM, Bao FL, Wei YH, Wang HY (2019) Polyhedron 160:207–212
Liu HY, Gao GM, Liu J, Wang HY (2018) Polyhedron 152:11–16
Liu J, Wei Y, Bao F, Li G, Liu H, Wang H (2019) Polyhedron 169:58–65
Wang HY, Liu HY (2017) Trans Met Chem 42:165–173
Tang K, Yun R, Lu Z, Du L, Zhang M, Wang Q, Liu H (2013) Crystal Growth Des 13:1382–1385
Zhang M, Wang Q, Lu Z, Liu H, Liu W, Bai J (2014) CrystEngComm 16:6287–6290
Boudreaux EA, Mulay LN (1976) Theory and application of molecular paramagnetism. Wiley, New York
Bruker AXS Inc (2003) SAINT (Version 6.45) and SADABS (Version 2.10); Bruker AXS Inc Madison, WI
Sheldrick GM (2008) Acta Crystallogr Sect A 64:112–122
Van Der Sluis P, Spek AL (1990) Acta Crystallogr Sect A 46:194–201
Yang F, Liang B, Zhang JH, Wu WH, Zheng SQ, Mao YY, Chen JQ, Cao JX, Zhu GZ (2018) Transit Met Chem 43:211–220
Gusev AN, Shul’gin VF, Beyjyyev E, Alexandrov GG, Eremenko IL, Linert W (2015) Polyhedron 85:525–529
Thompson LK (2002) Coord Chem Rev 233–234:193–206
Zhu HL, Zheng LM, Fu DG, Huang P, Bu WM, Tang WX (1999) Inorg Chim Acta 287:52–6011
Zhu HL, Cheng DY, Zheng YQ (2012) Inorg Chim Acta 388:37–45
Mohanta S, Nanda KK, Werner R, Haase W, Mukherjee AK, Dutta SK (1997) Nag K 36:4656–4664
Du M, Bu XH, Huang Z, Chen ST, Guo YM (2003) Inorg Chem 42:552–559
Kavlakoglu E, Elmali A, Elerman Y, Svoboda I (2002) Polyhedron 21:1539–1545
Acevedo-Cháveza R, Costas ME (1999) Polyhedron 18:1549–1553
Feng X, Wang LY, Zhao JS, Liu B, Wang JG, Shi XG (2009) Inorg Chim Acta 362:5127–5132
Acknowledgements
This work was supported by the Foundation of Jiangsu Students’ Innovation and Entrepreneurship Training Program (No. 201910320016Z), the Natural Science Foundation of Xuzhou City (KC19050), Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP) and PAPD of Jiangsu Higher Education Institution.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Di, L., Dong, H., Chen, D.N. et al. A copper-based coordination polymer formed through synergistic bridging of 1,2,4-triazole and acetate anions: synthesis, crystal structure and magnetic properties. Transit Met Chem 46, 57–63 (2021). https://doi.org/10.1007/s11243-020-00421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00421-9