Abstract
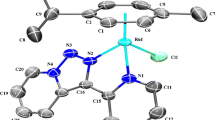
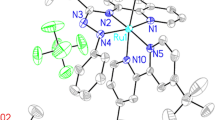
The Rh(III) complexes of 1-alkyl-2-{(o-thioalkyl)phenylazo}imidazole (SRaaiNR′, 1; R = R′= Me (a); R = Me, R′= Et (b); R = Et, R′= Me (c); R = R′= Et (d)), [Rh(SRaaiNR′)(PPh3)Cl2](ClO4) (2) have been synthesized. The complexes have been characterized by physicochemical and spectroscopic methods. The single-crystal X-ray diffraction study authenticates the structure of [Rh(SMeaaiNEt)(PPh3)Cl2](ClO4) (2b). The DNA binding ability of the complexes has been investigated by electronic absorption and fluorescence spectroscopic methods. Density functional theory computation technique has been used to enlighten the electronic structures and their spectral properties.
Graphic abstract
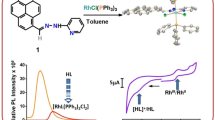
1-Alkyl-2-{(o-thioalkyl)phenylazo}imidazole complexes of Rh(III), [Rh(SRaaiNR′)(PPh3)Cl2](ClO4)](2) are synthesized and characterized. [Rh(SMeaaiNEt)(PPh3)Cl2](ClO4) (2b) is characterized by single-crystal X-ray diffraction study. Ligand acts as tridentate N(imidazole), N(azo) and S(thioether) donor centers. The DNA binding ability of the complexes is investigated by electronic absorption and fluorescence spectroscopic methods. The electronic structure and observed electronic transitions are interpreted by DFT and TDDFT computation.









Similar content being viewed by others
References
Yousefi R, Aghevlian S, Mokhtari F, Samouei H, Rashidi M, Nabavizadeh SM, Tavaf Z, Pouryasin Z, Niazi A, Faghihi R (2012) Appl Biochem Biotech 167(4):861–872
Kostova I (2006) Curr Med Chem 13(9):1085–1107
Muhammad N, Sadia N, Zhu C, Luo C, Guo Z, Wang X (2017) Chem Comm 53(72):9971–9974
Nagaj J, Kołkowska P, Bykowska A, Komarnicka UK, Kyzioł A, Jeżowska-Bojczuk M (2015) Med Chem Res 24:115–123
Heydari M, Moghadam ME, Tarlani A, Farhangian H (2017) Appl Biochem Biotech 182(1):110–127
Gasser G, Ott I, Metzler-Nolte N (2011) J Med Chem 54(1):3–25
Johnstone TC, Suntharalingam K, Lippard SJ (2016) Chem Rev 116(5):436–3486
Mukhopadhyay S, Gupta RK, Paitandi RP, Rana NK, Sharma G, Koch B, Rana LK, Hundal MS, Pandey DS (2015) Organometallics 34(18):4491–4506
Patel M, Chhasatia M, Bhatt B (2011) Med Chem Res 20(2):220–230
Arthi P, Shobana S, Srinivasan P, Mitu L, Rahiman AK (2015) Spectrochim Acta A 143:49–58
Kostrhunova H, Florian J, Novakova O, Peacock AFA, Sadler PJ, Brabec V (2008) J Med Chem 51:3635–3643
Cutillas N, Yellol GS, de Haro C, Vicente C, Rodríguez V, Ruiz J (2013) Coord Chem Rev 257:2784–2797
Sathyadevi P, Krishnamoorthy P, Butorac RR, Cowley AH, Dharmaraj N (2012) Metallomics 4:498–511
Aird RE, Cummings J, Ritchie AA, Muir M, Morris RE, Chen H, Sadler PJ, Jodrell DI (2002) Br J Cancer 86:1652–1657
Loughrey BT, Healy PC, Parsons PG, Williams ML (2008) Inorg Chem 47:8589–8591
Mendoza-Ferri MG, Hartinger CG, Mendoza MA, Groessl M, Egger AE, Eichinger RE, Mangrum JB, Farrell NP, Maruszak M, Bednarski PJ (2009) J Med Chem 52:916–925
Petrini A, Pettinari R, Marchetti F, Pettinari C, Therrien B, Galindo AN, Scopelliti R, Riedel T, Dyson PJ (2017) Inorg Chem 56:13600–13612
Payne R, Govender P, Therrien B, Clavel CM, Dyson PJ, Smith GS (2013) J Organomet Chem 729:20–27
Almodares Z, Lucas SJ, Crossley BD, Basri AM, Pask CM, Hebden AJ, Phillips RM, McGowan PC (2014) Inorg Chem 53:727–736
Banerjee D, Ray U, Jasimuddin S, Liou J-C, Lu T-H, Sinha C (2006) Polyhedron 25:1299–1306
Vogel AI, Tatchell AR, Furnis BS, Hannaford AJ, Smith PWG (1996) A text book of practical organic chemistry, 5th edn. Prentice Hall, Upper Saddle River
Sheldrick GM (1997) SHELXS-97. Program for the solution of crystal structure. University of Gottingen, Gottingen
Farrugia LJ (1997) ORTEP-3 for windows. J Appl Cryst 30:565
Spek AL (1999) PLATON. Molecular Geometry Program, University of Utrecht, Utrecht
Schlegel HB et al (2009) Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
EMSL, basis set library available http://www.emsl.pnl.gov/forms/basisform.html
Benesi HA, Hildebrand JH (1949) J Am Chem Soc 71:2703–2707
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Kluwer Academic/Plenum Publishers, New York
Valeur B (2001) Molecular fluorescence. Principles and applications. Wiley, Weinheim
Eftink MR, Ghiron CA (1981) Fluorescence quenching studies with proteins. Anal Biochem 114:199–227
Sardar D, Datta P, Saha R, Raghavaiah P, Sinha C (2013) J Organomet Chem 732:109–115
Pyle AM, Rehmann JP, Meshoyrer R, Kumar CV, Turro NJ, Barton JK (1989) J Am Chem Soc 111:3051–3058
García-Giménez JL, González-Álvarez M, Liu-González Malva, Macías B, Joaquín Borrás J, Alzuet G (2009) J Inorg Biochem 103:923–934
Acknowledgements
Financial support from the University Grant Commission (Reference No. F.: PSW-044/14-15 (ERO)), New Delhi, is gratefully acknowledged. Author P. Datta is thankful to TEQIP Phase-II, RCC Institute of Information Technology for financial support. We are also thankful to Dr. Suvendu Maity, D. S. Kothari Post Doctoral Fellow, Jadavpur University, for his support in X-ray structure analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11243_2020_414_MOESM1_ESM.doc
Supplementary material includes the experimental section, energy and composition of the selected frontier molecular orbitals calculated by the DFT, electronic transition data from the TDDFT calculation, 1H NMR and IR spectra, and some figures related to the DNA binding study (DOC 12557 kb)
Rights and permissions
About this article
Cite this article
Sardar, D., Datta, P. & Sinha, C. Rhodium(III) complexes of 1-Alkyl-2-{(o-thioalkyl) phenylazo}imidazoles: synthesis, structure, spectral characterization, DNA binding study and DFT calculation. Transit Met Chem 45, 595–603 (2020). https://doi.org/10.1007/s11243-020-00414-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00414-8