Abstract
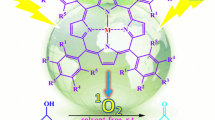
The aerobic oxidation of a variety of aromatic aldehydes to the corresponding carboxylic acids by molecular oxygen in the presence of 4-carboxyl tetraphenylporphyrin (H2TCPP), methylene blue (MB), cobalt(II) phthalocyanine sulfonate (CoPcS) and FeTCPPCl as water-soluble photosensitizers in organic-water biphasic media at room temperature under either visible light or sunlight is described. The products were obtained with 25–100% conversion and 100% selectivity. This method has a wide range of applicabilities, has a straightforward workup procedure, is chemoselective and proceeds under mild reaction conditions. The resulting products were obtained in good yields in reasonable times.





Similar content being viewed by others
References
Hollingworth GJ, Katritzky AR, Meth-Cohn O, Rees CW, Pattenden G (1995) In comprehensive organic functional group transformations. Elsevier Sci, Oxford
Hudlicky M (1990) Oxidations in organic chemistry. American Chemical Society, Washington DC
Larock RC (1999) Comprehensive organic transformations: a guide to functional group preparations, 2nd edn. Wiley, New York
Smith MB, March J (2001) March’s advanced organic chemistry: reactions mechanisms and structure, 5th edn. Wiley, New York
Bowden K, Heilbron IM, Jones ERH, Weedon BCL (1946) J Chem Soc. https://doi.org/10.1039/JR9460000039
Heilbron I, Jones E, Sondheimer F (1949) J Chem Soc. https://doi.org/10.1039/JR9490000604
Bladon P, Fabian JM, Henbest H, Koch H, Wood GW (1951) J Chem Soc. pp 2402–2411
Curtis R, Heilbron I, Jones E, Woods GF (1953) J Chem Soc. https://doi.org/10.1039/JR9530000457
Bowers A, Halsall T, Jones E, Lemin A (1953) J Chem Soc. https://doi.org/10.1039/JR9530002548
Djerassi C, Engle R, Bowers A (1956) J Org Chem 21:1547–1549
Benjamin RT, Sivakumar M, Hollist GO, Borhan B (2003) Org Lett 5:1031–1034
Nwaukwa SO, Keehn PM (1982) Tetrahedron Lett 23:3131–3134
Ganem B, Heggs RP, Biloski AJ, Schwartz DR (1980) Tetrahedron Lett 21:685–688
Joseph JK, Jain SL, Sain B (2007) Catal Commun 8:83–87
Lim M, Yoon CM, An G, Rhee H (2007) Tetrahedron Lett 48:3835–3839
Sloboda-Rozner D, Neimann K, Neumann R (2007) J Mol Catal A Chem 262:109–113
Mukhopadhyay C, Datta A (2008) Catal Commun 9:2588–2592
Uyanik M, Ishihara K (2009) Chem Commun. https://doi.org/10.1039/B823399C
Han A-R, Jeong YJ, Kang Y, Lee JY, Seo MS, Nam W (2008) Chem Commun. https://doi.org/10.1039/B716558G
Ellis PE Jr, Lyons JE (1990) Coord Chem Rev 105:181–193
Haranaka M, Hara A, Ando W, Akasaka T (2009) Tetrahedron Lett 50:3585–3587
Khavasi HR, Safari N (2004) J Mol Catal A 220:127–132
DeRosa MC, Crutchley RJ (2002) Coord Chem Rev 233:351–371
Greer A (2006) Acc Chem Res 39:797–804
Redmond RW, Gamlin JN (1999) Photochem Photobiol 70:391–475
Bonnett R (1995) Chem Soc Rev 24:19–33
Weber L, Hommel R, Behling J, Haufe G, Hennig H (1994) J Am Chem Soc 116:2400–2408
Pandey R, Zheng G (2000) The porphyrin handbook. In: Kadish KM, Smith KM, Guilard R (eds), vol 6. Academic Press, Boston, pp 157–230
Pushpan S, Venkatraman S, Anand V, Sankar J, Parmeswaran D, Ganesan S, Chandrashekar T (2002) Curr Med Chem Anti-Cancer Agents 2:187–207
Nyman ES, Hynninen PH (2004) J Photochem Photobiol B 73(1–2):1–28
Sorokin AB (2013) Chem Rev 113:8152–8191
Vashurin A, Maizlish V, Kuzmina I, Znoyko S, Morozova A, Razumov M, Koifman O (2017) J Porphyr Phthalocyanines 21:37–47
Ebrahimian Pirbazari A (2015) Procedia Mater Sci 11:622–627
Wang D, Guo R, Wang S, Liu F, Wang Y, Zhao C (2016) Desalin Water Treat 57:25226–25234
Hajimohammadi M, Bahadoran F, Davarani SSH, Safari N (2010) React Kinet Mech Cat 99:243–250
Hajimohammadi M, Safari N, Mofakham H, Deyhimi F (2011) Green Chem 13:991–997
Kalajahi SSM, Hajimohammadi M, Safari N (2014) React Kinet Mech Cat 113:629–640
Hajimohammadi M, Ghasemi H (2016) J Porphyr Phthalocyanines 20:670–676
Adler AD, Longo FR, Shergalis W (1964) J Am Chem Soc 86:3145–3149
Yan GP, Bischa D, Bottle SE (2007) Free Rad Biol Med 43:111–116
Kulinich VP, Shaposhnikov GP, Badaukaite RA (2010) Macroheterocycles 3:23–29
Knör G (2001) Chem Bio Chem 2:593–596
Staicu A, Pascu A, Nuta A, Sorescu A, Raditoiu V, Pascu ML (2013) Rom Rep Phys 65:1032–1051
Sawyer DT (1991) Oxygen chemistry. Oxford University Press, Oxford
Min DB, Boff JM (2002) Compr Rev Food Sci Food Saf 1:58–72
Aubry JM, Pierlot C, Rigaudy J, Schmidt R (2003) Acc Chem Res 36:668–675
Nowakowska M (1978) Macromol Chem Phys 179:2959–2967
Nowakowska M (1980) Macromol Chem Phys 181:1021–1027
Olea AF, Wilkinson F (1995) J Phys Chem 99:4518–4524
Harbour JR, Issler SL (1982) J Am Chem Soc 104:903–905
Chen Y, Xu S, Li L, Zhang M, Shen J, Shen T (2001) Dyes Pigments 51:63–69
Bressan M, Morvillo A (1989) Inorg Chem 28:950
Toffoli DJ, Gomes L, Junior NDV, Courrol LC (2008) In: AIP conference proceedings, AIP, vol 992. p 1207
Bernini R, Coratti A, Provenzano G, Fabrizi G, Tofani D (2005) Tetrahedron Lett 61:1821–1825
Wu XA, Ying P, Liu JY, Shen HS, Chen Y, He L (2009) Synth Commun 39:3459–3470
Nield E, Stephens R, Tatlow JC (1959) J Chem Soc. https://doi.org/10.1039/JR9590000166
Donleavy JJ (1936) J Am Chem Soc 58:1004–1005
Ueda I (1975) Bull Chem Soc Jpn 48:2306–2309
Taha N, Chidambaram M, Dakka J, Sasson Y (2009) Catal Lett 129:358–362
Ferenc WI, Walkow-Dziewulska AG (2001) J Serb Chem Soc 66:543–554
Iqbal N, Choi S, You Y, Cho EJ (2013) Tetrahedron Lett 54:6222–6225
Acknowledgements
Financial support of this work by Iran National Science Foundation (INSF) no. 96005616, and Research Council of Kharazmi University are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajimohammadi, M., Ahmadi Khamesi, Z. & Nosrati, P. Efficient aerobic photooxygenation of aldehydes to carboxylic acids using cobalt(II) phthalocyanine sulfonate as a photosensitizer in organic-water biphasic media. Transit Met Chem 44, 167–173 (2019). https://doi.org/10.1007/s11243-018-0281-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0281-x