Abstract
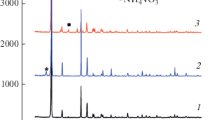
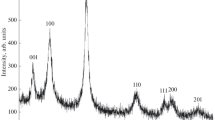
The reduction of vanadium pentoxide with formamide under hydrothermal–microwave treatment at 180–210 °C allows for the selective synthesis of ammonium hexavanadate(V) (NH4)2V6O16, ammonium trivanadate(IV,V) (NH4)2V3O8 or ammonium vanadate(IV,V) with the hypothesized composition of (NH4)0.22V2O5. An increase in both the temperature of the hydrothermal reaction and the molar ratio of the reagents (HC(O)NH2:V2O5) promotes the reduction of vanadium(V) to vanadium(IV). The synthesis is time-saving and provides a sufficiently high yield of single-phase reaction products (up to 60–70%). Our data call into question the existence of a recently reported crystalline ammonium tetravanadate(IV), (NH4)2V4O9.



Similar content being viewed by others
References
Bahlawane N, Lenoble D (2014) Vanadium oxide compounds: structure, properties, and growth from the gas phase. Chem. Vap. Depos. 20:299–311. https://doi.org/10.1002/cvde.201400057
Kang Y-B (2012) Critical evaluation and thermodynamic optimization of the VO–VO2.5 system. J Eur Ceram Soc 32:3187–3198. https://doi.org/10.1016/j.jeurceramsoc.2012.04.045
Morin FJ (1959) Oxides which show a metal-to-insulator transition at the neel temperature. Phys Rev Lett 3:34–36. https://doi.org/10.1103/PhysRevLett.3.34
Coy H, Cabrera R, Sepúlveda N, Fernández FE (2010) Optoelectronic and all-optical multiple memory states in vanadium dioxide. J Appl Phys 108:113115. https://doi.org/10.1063/1.3518508
Oka Y, Yao T, Yamamoto N (1990) Powder X-ray crystal structure of VO2(A). J Solid State Chem 86:116–124. https://doi.org/10.1016/0022-4596(90)90121-D
Théobald F, Cabala R, Bernard J (1976) Essai sur la structure de VO2(B). J Solid State Chem 17:431–438. https://doi.org/10.1016/S0022-4596(76)80013-8
Hagrman D, Zubieta J, Warren CJ, Meyer LM, Treacy MMJ, Haushalter RC (1998) A new polymorph of VO2 prepared by soft chemical methods. J Solid State Chem 138:178–182. https://doi.org/10.1006/jssc.1997.7575
Liu L, Cao F, Yao T, Xu Y, Zhou M, Qu B, Pan B, Wu C, Wei S, Xie Y (2012) New-phase VO2 micro/nanostructures: investigation of phase transformation and magnetic property. New J Chem 36:619–625. https://doi.org/10.1039/C1NJ20798A
Wu C, Hu Z, Wang W, Zhang M, Yang J, Xie Y (2008) Synthetic paramontroseite VO2 with good aqueous lithium–ion battery performance. Chem Commun 1:1. https://doi.org/10.1039/b806009f
Li M, Magdassi S, Gao Y, Long Y (2017) Hydrothermal synthesis of VO2 polymorphs: advantages, challenges and prospects for the application of energy efficient smart windows. Small 13:1701147. https://doi.org/10.1002/smll.201701147
Post K, Robins RG (1976) Thermodynamic diagrams for the vanadium-water system at 298.15K. Electrochim Acta 21:401–405. https://doi.org/10.1016/0013-4686(76)85115-8
Dong B, Shen N, Cao C, Chen Z, Luo H, Gao Y (2016) An intermediate phase (NH4)2V4O9 and its effects on the hydrothermal synthesis of VO2(M) nanoparticles. CrystEngComm 18:558–565. https://doi.org/10.1039/C5CE02004B
Luskan K, Gyrenko A, Bubel T, Mysov O (2017) Synthesis and physicochemical properties of ammonium tetravanadate for obtaining VO2. Chem. Chem. Technol. 11:247–252. https://doi.org/10.23939/chcht11.02.247
Leonardi SG, Primerano P, Donato N, Neri G (2013) Behavior of sheet-like crystalline ammonium trivanadate hemihydrate (NH4V3O8 × 0.5H2O) as a novel ammonia sensing material. J Solid State Chem 202:105–110. https://doi.org/10.1016/j.jssc.2013.03.028
Mai LQ, Lao CS, Hu B, Zhou J, Qi YY, Chen W, Gu ED, Wang ZL (2006) Synthesis and electrical transport of single-crystal NH4V3O8 nanobelts. J. Phys. Chem. B. 110:18138–18141. https://doi.org/10.1021/jp0645216
Hine J, King RSM, Midden WR, Sinha A (1981) Hydrolysis of formamide at 80 °C and pH 1–9. J Org Chem 46:3186–3189. https://doi.org/10.1021/jo00329a007
Miyakawa S, James Cleaves H, Miller SL (2002) The cold origin of life: A. Implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. Orig Life Evol Biosph 32:195–208. https://doi.org/10.1023/A:1016514305984
Okazaki M, Funazukuri T (2006) Decomposition of acetamide and formamide in pressurized hot water. J Mater Sci 41:1517–1521. https://doi.org/10.1007/s10853-006-4616-1
Gordon L (1952) Precipitation from homogeneous solution. Anal Chem 24:459–462. https://doi.org/10.1021/ac60063a006
Štengl V, Šubrt J, Bezdička P, Maříková M, Bakardjieva S (2003) Homogenous precipitation with urea—an easy process for making spherical hydrous metal oxides. Solid State Phenom 90–91:121–128. https://doi.org/10.4028/www.scientific.net/SSP.90-91.121
Matijević E (1993) Preparation and properties of uniform size colloids. Chem Mater 5:412–426. https://doi.org/10.1021/cm00028a004
Garg A, Matijević E (1988) Preparation and properties of uniform coated inorganic colloidal particles. J Colloid Interface Sci 126:243–250. https://doi.org/10.1016/0021-9797(88)90118-X
Yasukawa A, Takase H, Kandori K, Ishikawa T (1994) Preparation of calcium hydroxyapatitie using amides. Polyhedron 13:3071–3078. https://doi.org/10.1016/S0277-5387(00)83673-6
Manohara GV, Kunz DA, Kamath PV, Milius W, Breu J (2010) Homogeneous precipitation by formamide hydrolysis: synthesis, reversible hydration, and aqueous exfoliation of the layered double hydroxide (LDH) of Ni and Al. Langmuir 26:15586–15591. https://doi.org/10.1021/la103108f
Geng F, Matsushita Y, Ma R, Xin H, Tanaka M, Iyi N, Sasaki T (2009) Synthesis and properties of well-crystallized layered rare-earth hydroxide nitrates from homogeneous precipitation. Inorg Chem 48:6724–6730. https://doi.org/10.1021/ic900669p
Range K-J, Eglmeier C, Heyns AM, de Waal D (1990) Ammonium hexavanadate, (NH4)2V6O16: preparation, crystal structure, infrared spectra and high-pressure reactions. Z Naturforsch 45:31–38
Zavalij PY, Whittingham MS (1999) Structural chemistry of vanadium oxides with open frameworks. Acta Crystallogr Sect B: Struct Sci 55:627–663. https://doi.org/10.1107/S0108768199004000
Rumble JR (ed) (2017) CRC handbook of chemistry and physics, 98th edn. CRC Press LLC, Boca Raton
Dell’Agli G, Grippo SM, Mascolo G (1993) Thermal behaviour of (NH4)2V6O16 prepared by hydrothermal crystallization. Thermochim Acta 227:197–204. https://doi.org/10.1016/0040-6031(93)80262-9
Wriedt HA (1989) The O–V (oxygen–vanadium) system. Bull. Alloy Phase Diagrams. 10:271–277. https://doi.org/10.1007/BF02877512
Katzke H, Tolédano P, Depmeier W (2003) Theory of morphotropic transformations in vanadium oxides. Phys. Rev. B. 68:24109. https://doi.org/10.1103/PhysRevB.68.024109
Ren T-Z, Yuan Z-Y, Zou X (2007) Crystal growth of mixed-valence ammonium vanadates. Cryst Res Technol 42:317–320. https://doi.org/10.1002/crat.200610821
Theobald FR, Theobald J-G, Vedrine JC, Clad R, Renard J (1984) Crystal growth, structure, electron paramagnetic resonance and magnetic properties of (NH4)2V3O8. J Phys Chem Solids 45:581–587. https://doi.org/10.1016/0022-3697(84)90050-7
Zakharova GS, Enyashin AN, Podval’naya NV, Zhuravlev NA, Kuznetsov MV, Gorodetsky RS, Liu Y, Zhu Q (2017) Structural, electronic properties of microscale (NH4)2V3O8 fabricated using a novel preparation method. J Phys Chem Solids 101:58–64. https://doi.org/10.1016/j.jpcs.2016.10.011
Xu G, He H, Wan H, Liu R, Zeng X, Sun D, Huang X, Wang H (2016) Facile synthesis and lithium storage performance of (NH4)2V3O8 nanoflakes. J Appl Electrochem 46:879–885. https://doi.org/10.1007/s10800-016-0973-x
Tudo J, Jolibois B (1969) Étude des phases isolées lors de la reduction de V2O5 par l’hydrogène naissant, en présence d’ammonium. C. R. Hebd. Seances Acad. Sci Ser. C. 269:1112–1115
Dong B, Shen N, Cao C, Chen Z, Luo H, Gao Y (2016) Phase and morphology evolution of VO2 nanoparticles using a novel hydrothermal system for thermochromic applications: the growth mechanism and effect of ammonium (NH4 +). RSC Adv. 6:81559–81568. https://doi.org/10.1039/C6RA14569H
Jia B-R, Qin M-L, Zhang Z-L, Li S-M, Wang X-L, Huang M, Wu H-Y, Chen Z, Lu X, Zhang L, Qu X-H (2017) Square-nanosheet flowers with an ammonium vanadate phase and their transformation to VO2(B) net-like nanosheets. J. Alloys Compd. 704:79–88. https://doi.org/10.1016/j.jallcom.2017.02.046
Ma Y, Ji S, Zhou H, Zhang S, Li R, Zhu J, Li W, Guo H, Jin P (2015) Synthesis of novel ammonium vanadium bronze (NH4)0.6V2O5 and its application in Li-ion battery. RSC Adv 5:90888–90894. https://doi.org/10.1039/c5ra18074k
Ma Y, Zhou H, Zhu J, Ji S, Bao S, Li R, Jin P (2016) Synthesis of flake-like VO2(M) by annealing a novel (NH4)0.6V2O5 phase and its thermochromic characterization. Ceram Int 42:16382–16386. https://doi.org/10.1016/j.ceramint.2016.07.030
Yuan R-M, Fu G, Xu X, Wan H-L (2011) Mechanisms for Selective Catalytic Oxidation of Ammonia over Vanadium Oxides. J Phys Chem C 115:21218–21229. https://doi.org/10.1021/jp206265p
Acknowledgements
The authors express sincere gratitude to Dr. N.P. Simonenko (IGIC RAS) for the thermal analysis of samples. The work was supported by RFBR Grant 17-03-01157 and performed using the equipment of the Joint Research Center for Physical Methods of Research of IGIC RAS (JRC PMR IGIC RAS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teplonogova, M.A., Yapryntsev, A.D., Baranchikov, A.E. et al. Selective hydrothermal synthesis of ammonium vanadates(V) and (IV,V). Transit Met Chem 44, 25–30 (2019). https://doi.org/10.1007/s11243-018-0265-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0265-x