Abstract
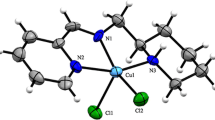
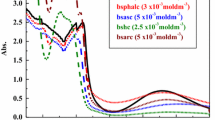
The biological activities of two binuclear copper(II) complexes containing S-alkenyl derivatives of thiosalicylic acid are reported [alkenyl = propenyl (L1), isobutenyl (L2)]. The structure of the complex with the S-isobutenyl derivative (C2) was confirmed by single-crystal X-ray structure analysis, which revealed that the structure consists of centrosymmetric, dinuclear complex molecules [Cu2(S-i-butenyl-thiosal)4(DMSO)2] containing two Cu(II) centers bridged by four S-isobutyl-thiosalicylate ligands in a paddle-wheel type structure. The Cu(II) atom is situated in a distorted square-pyramidal environment formed by carboxylate oxygen atoms in the basal plane and a DMSO ligand in the axial position. The reactivities of the complexes toward guanosine-5′-monophosphate (5′-GMP) were investigated. Complex C2 ([Cu2(S-i-butenyl-thiosal)4(H2O)2]) reacted more rapidly with 5′-GMP than complex C1. The interactions of complexes C1 and C2 with calf thymus DNA (CT-DNA) were examined by absorption (UV–Vis) and emission spectral studies (ethidium bromide displacement studies), revealing good DNA interaction abilities. The antimicrobial activities of the free ligands and their complexes were tested by microdilution method, and both minimal inhibitory and microbicidal concentrations were determined. All the tested substances demonstrated selective and moderate antibacterial activity on gram-positive bacteria, but low antibacterial activity on gram-negative bacteria. Also, the tested substances demonstrated low antifungal activity.









Similar content being viewed by others
References
Tisato F, Marzano C, Porchia M, Pellei M, Santini C (2010) Med Res Rev 30:708–749
Marzano C, Pellei M, Tisato F, Santini C (2009) Anticancer Agents Med Chem 9:185–211
Prudhomme M (2013) Advances in anticancer agents in medicinal chemistry. Bentham Science Publishers, Clermont-Ferrand
Gielen M, Tiekink ER (2005) Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. Wiley, Chichester
Goodman VL, Brewer GJ, Merajver SD (2004) Endocr Relat Cancer 11:255–263
Iakovidis I, Delimaris I, Piperakis SM (2011) Mol Biol Int 2011:1–13
H. Jacobelli, US Patent 20050267095
Reif S, Weis B, Aeed H et al (1999) J Hepatol 31:1053–1061
Serrano G, Bonillo J, Aliaga A et al (1990) J Am Acad Dermatol 23:479–483
Ferrer EG, Williams PA (1997) Polyhedron 16:3323–3325
Wehr-Candler T, Henderson W (2016) Coord Chem Rev 313:111–155
Gilbert JG, Addison AW, Nazarenko AY, Butcher RJ (2001) Inorg Chim Acta 324:123–130
Nikolić MV, Mijajlović MŽ, Jevtić VV et al (2014) Polyhedron 79:80–87
Nikolić MV, Mijajlović MŽ, Jevtić VV et al (2016) J Mol Struct 1116:264–271
Chohan ZH, Shad HA, Youssoufi MH, Hadda TB (2010) Eur J Med Chem 45:2893–2901
Patil SA, Naik VH, Kulkarni AD, Badami PS (2010) Spectrochim Acta A Mol Biomol Spectrosc 75:347–354
Shebl M, Khalil SM, Ahmed SA, Medien HA (2010) J Mol Struct 980:39
Katsarou ME, Efthimiadou EK, Psomas G, Karaliota A, Vourloumis D (2008) J Med Chem 51:470–478
Siddiqi ZA, Khalid M, Kumar S, Shahid M, Noor S (2010) Eur J Med Chem 45:264–269
Betanzos-Lara S, Gracia-Mora I, Granada-Macyas P, Flores-Alamo M, Barba-Behrens N (2013) Inorg Chim Acta 397:94–100
Abou-Hussein AA, Linert W (2012) Spectrochim Acta Part A Mol Biomol Spectrosc 95:596–609
Amer S, El-Wakiel N, El-Ghamry H (2013) J Mol Struct 1049:326–335
Creaven BS, Egan DA, Karcz D (2007) J Inorg Biochem 101:1108–1119
Efthimiadou EK, Katsarou ME, Karaliota A, Psomas G (2008) J Inorg Biochem 102:910–920
El-Gamel NE, Zayed MA (2011) Spectrochim Acta Part A 82:414–423
Geeta B, Shravankumar K, Muralidhar Reddy P (2010) Spectrochim Acta Part A 77:911–915
Siddiqi ZA, Sharma PK, Shahid M, Khalid M, Siddique A, Kumar S (2012) Eur J Med Chem 57:102–111
Chalkidou E, Perdih F, Turer I, Kessissoglou PD, Psomas G (2012) J Inorg Biochem 113:55–65
Bukonjić AM, Tomović DL, Nikolić MV et al (2017) J Mol Struct 1128:330–337
Radić GP, Glođović VV, Radojević ID et al (2012) Polyhedron 31:69–76
Tomović DL, Bukonjić AM, Kočović A et al (2017) Serb J Exp Clin Res. 18:13–18
Agilent, CrysAlis PRO (2014) PRO. England, Agilent Technologies, Yarnton, Oxfordshire
Burla MC, Camalli M, Carrozzini B et al (2003) J Appl Crystallogr 36:1103
Sheldrick GM (2008) Acta Crystallogr A 64:112–122
Nardelli M (1995) J Appl Crystallogr 28:659
Spek AL (2003) J Appl Crystallogr 36:7–13
Macrae CF, Edgington PR, McCabe P et al (2006) J Appl Crystallogr 39:453–457
Meadows KA, Liu F, Sou J, Hudson BP, McMillin DR (1993) Inorg Chem 32:2919–2923
Andrews JM (2005) J Antimicrob Chemoth 56:60–76
Sarker SD, Nahar L, Kumarasamy Y (2007) Methods 42:321–324
Van Niekerk JN, Schoening FR (1953) Acta Crystallogr 6:227–232
Reyes-Ortega Y, Alcantara-Flores JL, Hernandez-Galindo MC (2005) J Am Chem Soc 127:16312–16317
Long EC, Barton JK (1990) Acc Chem Res 23:271–273
Pasternack RF, Gibbs EJ, Villafranca JJ (1983) J Biochem 22:251
Koumousi ES, Zampakou M, Raptopoulou CP et al (2012) Inorg Chem 51:7699–7710
Rizvi MA, Zaki M, Afzal M et al (2015) Eur J Med Chem 90:876–888
Meyer-Almes FJ, Porschke D (1993) Biochemistry 32:4246–4253
Liu ZC, Wang BD, Yang ZY, Li Y, Qin DD, Li TR (2009) Eur J Med Chem 44:4477–4484
Howe GM, Wu KC, Bauer WR (1976) Biochemistry 19:4339–4346
Li DD, Tian JL, Gu W, Liu X, Yan SP (2010) J Inorg Biochem 104:171–179
Jiang M, Li Y, Wu Z, Liu Z, Yan C (2009) J Inorg Biochem 103:833–844
Acknowledgements
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Projects 172016, 173032, 172034, 172035, 172011).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tomović, D.L., Bukonjić, A.M., Jevtić, V.V. et al. DNA binding, antibacterial and antifungal activities of copper(II) complexes with some S-alkenyl derivatives of thiosalicylic acid. Transit Met Chem 43, 137–148 (2018). https://doi.org/10.1007/s11243-018-0201-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0201-0