Abstract
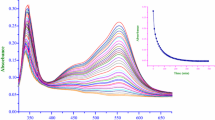
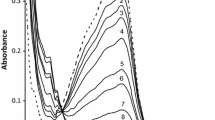
The kinetics and mechanism of base hydrolysis of tris(3-(2-pyridyl)-5,6-bis(4-phenyl sulphonic acid)-1,2,4-triazine)iron(II), \({\text{Fe}}({\text{PDTS}})_{3}^{4 - }\) have been studied in aqueous, sodium dodecyl sulphate (SDS) and cetyltrimethyl ammonium bromide (CTAB) media at 25, 35 and 45 °C under pseudo-first-order conditions, i.e. \(\left[ {\text{OH}^{ - } } \right]\) ≫ \({\text{Fe}}({\text{PDTS}})_{3}^{4 - }\). The reaction is first order each in \({\text{Fe}}({\text{PDTS}})_{3}^{4 - }\) and hydroxide ion. The rate increases with increasing ionic strength in aqueous and SDS media, whereas this parameter has little effect in CTAB. In SDS medium, the rate-determining step involves the reaction between \(\left[ {\text{OH}^{ - } } \right]\) and \({\text{Fe}}({\text{PDTS}})_{3}^{4 - }\), whereas in CTAB medium, it involves reaction between a neutral ion pair, {\({\text{Fe}}({\text{PDTS}})_{3}^{4 - }\)·4CTA+} and \(\left[ {\text{OH}^{ - } } \right]\) ions. The specific rate constants and thermodynamic parameters (E a, ΔH #, ΔS # and ΔG #35°C ) have been evaluated in all three media. The near equal values of ΔG #35°C obtained in aqueous and SDS media suggest that these reactions occur essentially by the same mechanism. Slightly lower ΔG #35°C values in CTAB medium can be attributed to a higher concentration of reactants in the Stern layer. The reaction is inhibited in SDS medium but catalysed in CTAB. The former can be attributed to the anionic surfactant creating more repellent space between the reactants. Catalysis in CTAB medium is ascribed to electrophilic and hydrophilic interactions between hydroxide ion/substrate with the cationic Stern layer, resulting in increased local concentrations of both reactants.




Similar content being viewed by others
References
Kundu A, Dasmandal S, Rudra S, Mahapatra A (2015) J Mol Liq 209:99–103
Abdel-Rahman RM, Makki MS, Ali TE, Ibrahim MA (2015) J Heterocycl Chem 52:1595–1607
Kundu A, Dasmandal S, Majumdar T, Mahapatra A (2014) Colloids Surf A Phys Chem Eng Asp 452:148–153
Mandal HK, Majumdar T, Mahapatra A (2011) Int J Chem Kinet 43:579–589
Gharib A, Ezz-Eldin A, Gosal N, Burgess J (2001) Croat Chem Acta 74:545–558
Mandal HK, Dasmandal S, Mahapatra A (2016) J Disp Sci Technol 37:555–564
Gibbs CR (1976) Anal Chem 48:1197–1201
Stookey LL (1970) Anal Chem 42:779–781
Ratnam S, Anipindi NR (2012) Trans Met Chem 37:453–462
Bellam R, Anipindi NR (2014) Trans Met Chem 39:311–326
Bellam R, Jaganyi D (2017) Int J Chem Kinet 49:182–196
Bellam R, Sivamadhavi S, Ramakrishna S, Mambanda A, Jaganyi D, Anipindi NR (2017) J Coord Chem 70:1893–1909
Burgess J, Hubbard CD, Miyares PH, Cole TL, Dasgupta TP, Leebert S (2005) Trans Met Chem 30:957–963
Baxendale J, George P (1950) Trans Faraday Soc 46:736–744
Baxendale J, George P (1950) Trans Faraday Soc 46:55–63
Krumholz P (1949) Nature 163:724–725
Lee T, Kolthoff I, Leussing D (1948) J Am Chem Soc 70:3596–3600
Bellam R, Raju GG, Anipindi NR, Jaganyi D (2016) Trans Met Chem 41:271–278
Bellam R, Anipindi NR (2012) Trans Met Chem 37:489–495
Origin 7.5™ SRO, v7.5714 (B5714) (2003) Origin Lab Corporation, Northampton One, Roundhouse Plaza, Northampton, MA, 01060 USA
Atwood JD (1997) Inorganic and organometallic reaction mechanisms. Whiley-VCH Inc, NY, pp 43–61
Laidler K (2003) Physical chemistry. Houghton Mifflin Company, Boston
Connors KA (1990) Chemical kinetics: the study of reaction rates in solution. VCH, New York
Evans MG, Polanyi M (1935) Trans Faraday Soc 31:875–894
Blandamer MJ, Burgess J, Wellings P (1979) Trans Met Chem 4:95–97
Desando M, Reeves L (1986) Can J Chem 64:1817–1822
Berezin IV, Martinek K, Yatsimirskii AK (1973) Russ Chem Rev 42:787–802
Eyring H (1935) J Chem Phys 3:107–115
Atwood JD (1997) Inorganic and organometallic reaction mechanisms, 2nd edn. Wiley, New York, pp 43–61
Gangwar S, Rafiquee M (2007) Int J Chem Kinet 39:638–644
Berezin IV, Martinek K, Yatsimirsky A (1973) Usp Khim 42:1729–1756
Menger FM, Portnoy CE (1967) J Am Chem Soc 89:4698–4703
Burgess J (1967) J Chem Soc A Inorg Phys Theor. doi:10.1039/J19670000431
Moroi Y, Braun AM, Graetzel G (1979) J Am Chem Soc 101:567–572
Holyer R, Hubbard C, Kettle S, Wilkins R (1966) Inorg Chem 5:622–625
Burgess J, Prince R (1963) J Am Chem Soc (Resumed) 12:5752–5758
Acknowledgements
The authors are gratefully indebted to the University Grants Commission, New Delhi, India, and University of KwaZulu-Natal, South Africa, for financial support to Rajesh Bellam.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellam, R., Anipindi, N.R. & Jaganyi, D. Base hydrolysis of tris(3-(2-pyridyl)-5,6-bis(4-phenyl sulphonic acid)-1,2,4-triazine)iron(II) in aqueous, SDS and CTAB media: kinetic and mechanistic study. Transit Met Chem 42, 719–725 (2017). https://doi.org/10.1007/s11243-017-0179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0179-z