Abstract
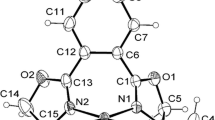
A Pd(II) complex of two sterically crowded ligands, specifically an N,S-donor thiosemicarbazone and an N-donor imidazole, has been synthesized and characterized by physicochemical and spectroscopic methods. X-ray single-crystal analysis revealed that the coordination geometry around the palladium center is distorted square planar, and the chloride ligand is involved in intermolecular bifurcated X–H···Y-type (where X = C, N and Y = Cl) hydrogen bonding. This complex proved to be a highly active and retrievable pre-catalyst for additive-free Suzuki–Miyaura cross-coupling reactions of arylboronic acids with aryl bromides or chlorides at room temperature and 60 °C, respectively. The reactions require a low catalyst loading and the complex is converted to ~1.5–2.0 nm-sized Pd nanoparticles (probably the real catalyst). The catalyst can be reused up to seven times without significant loss in activity. Since the reaction proceeds under mild conditions in aqueous medium and the catalyst is recoverable, it provides an environmentally benign alternative to the existing protocols for Suzuki–Miyaura reactions.
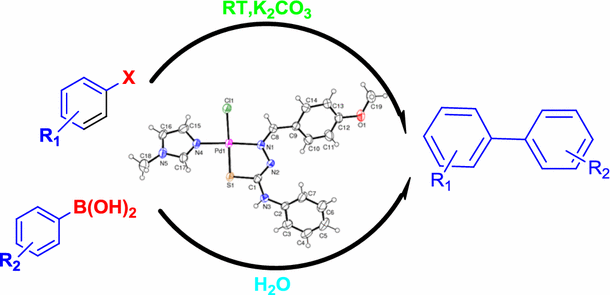
Graphical abstract
Biaryls can be synthesized in high yield under greener reaction conditions in the presence of efficient pre-catalyst, thiosemicarbazone–palladium(II)–imidazole.







Similar content being viewed by others
References
Miyaura N, Suzuki A (1979) J Chem Soc Chem Comm 19:866–867
Miyaura N, Yamadnandea K, Suzuki A (1979) Tetrahedron Lett 20:3437–3440
Suzuki A (2011) Angew Chem Int Ed 50:6722–6737
Fihri A, Bouhrara M, Nekoueishahraki B, Basset JM, Polshettiwar V (2011) Chem Soc Rev 40:5181–5203
Miyaura N, Suzuki A (1995) Chem Rev 95:2457–2483
Das P, Linert W (2016) Coord Chem Rev 311:1–23
Paul S, Islam MM, Islam SM (2015) RSC Adv 5:42193–42221
Begum T, Mondal M, Borpuzari MP, Kar R, Kalita G, Gogoi PK, Bora U (2017) Dalton Trans 46:539–546
Marziale AN, Faul SH, Reiner T, Schneider S, Eppinger J (2010) Green Chem 12:35–38
Sabounchei SJ, Ahmadi M, Panahimehr M, Bagherjeri FA, Nasri Z (2014) J Mol Catal A 249:383–384
Schaarschmidt D, Lang H (2011) ACS Catal. 1:411–416
Monnereau L, Mall HE, Semeril D, Matt D, Toupet L (2014) Eur J Inorg Chem 2014:1364–1372
Li JH, Liu WH (2004) J Org Chem 6:2809–2811
Blakemore JD, Chalkley MJ, Farnaby JH, Guard LM, Hazari N, Incarvito CD, Luzik ED, Suh HW (2011) Organometallics 30:1818–1829
Liu T, Zhao X, Shen Q, Lu L (2012) Tetrahedron 68:6535–6547
Susanto W, Chu CY, Ang WJ, Chou TC, Lo LC, Lam Y (2012) Green Chem 14:77–80
Mino T, Shirae Y, Sakamoto M, Fijita T (2005) J Org Chem 70:2191–2194
Xu C, Gong JF, Guo T, Zhang YH, Wu YJ (2008) J Mol Catal A Chem 279:69–76
Hanhan ME, Martinez-Manez R, Ros-Lis RJ (2012) Tetrahedron Lett 53:2388–2391
Kostas ID (2008) Inorg Chim Acta 361:1562–1565
Tenchiu AC, Ventouri IK, Ntasi G, Palles D, Kokotos G, Demertzi DK, Kostas ID (2015) Inorg Chim Acta 435:142–146
Verma PR, Mandal S, Gupta P, Mukhopadhyay P (2013) Tetrahedron Lett 54:4914–4917
Yan H, Chellan P, Li T, Mao J, Chibale K, Smith GS (2013) Tetrahedron Lett 54:154–157
Dutta J, Bhattacharya S (2013) RSC Advances 3:10707–10721
Dutta J, Datta S, Seth DK, Bhattacharya S (2012) RSC Adv 2:11751–11763
Paula P, Datta S, Haldera S, Acharyya R, Basuli F, Butcher RJ, Peng SM, Lee GH, Castineiras A, Drew MGB, Bhattacharya S (2011) J Mol Catal A Chem 344:62–73
Pandiarajan D, Ramesh R, Liu Y, Suresh R (2013) Inorg Chem Commun 33:33–37
Lobana TS, Kumari P, Hundal G, Butcher RJ, Castineiras A, Akitsu T (2013) Inorg Chim Acta 394:605–615
Pelosi G, Bisceglie F, Bignami Ronzi P, Schiavone P, Re MC, Casoli C, Pilotti E (2010) J Med Chem 53:8765–8769
Zhang WH, Chien SW, Hor TSA (2011) Coord Chem Rev 255:1991–2024
Kalinowski J, Fattori V, Cocchi M, Williams JAG (2011) Coord Chem Rev 255:2401–2425
Guerchais V, Fillaut JL (2011) Coord Chem Rev 255:2448–2457
Borah G, Boruah D, Sarmah G, Bharadwaj SK, Bora U (2013) Appl Organomet Chem 27:688–694
Dewan A, Bora U, Borah G (2014) Tetrahedron Lett 55:1689–1692
Gogoi A, Dewan A, Borah G, Bora U (2015) New J Chem 39:3341–3344
Borah G, Sarmah PP, Boruah D (2015) Bull Korean Chem Soc 36:1226–1230
Saikia B, Ali AA, Boruah PR, Sarma D, Barual NC (2015) New J Chem 39:2440–2443
Agarwala BV, Reddy PSN (1988) Trans Met Chem 13:187–189
Tang YQ, Lu M, Shao LX (2011) J Organomet Chem 696:3741–3744
Nakamoto K (1997) Infrared and raman spectra of inorganic and coordination compounds, 5th edn. Wiley, New York
Desiraju GR, Steiner T (1999) The weak hydrogen bond. Oxford University Press, Oxford
Freytag M, Jones PG (2000) Chem Commun 4:277–278
Fiedler D, Leung DH, Bergman RG, Raymond KN (2005) Acc Chem Res 38:351–360
Watson JD, Crick FHC (1953) Nature 171:737–738
Gogoi N, Begum T, Bora U, Gogoi PK (2015) RSC Adv 5:95344
Kitamura Y, Sako S, Tsutsui A, Monguchi Y, Maegawa T, Kitade Y, Sajiki H (2010) Adv Synth Catal 352:718–730
Pathak A, Singh AP (2016) J Porous Mater. doi:10.1007/s10934-016-0266-0
Yang S, Dong J, Yao Z, Shen C, Shi X, Tian Y, Lin S, Zhang X (2014) Sci Rep 4:4501
Jin Y, Zhao J, Li F, Jia W, Liang D, Chen H, Li R, Hu J, Ni J, Wu T, Zhong D (2016) Electrochim Acta 220:83–90
Acknowledgements
The authors acknowledge the analytical services provided by SAIF IISC, Bangalore; SAIF, CIL, Punjab University, Chandigarh; Department of Chemical Sciences, Tezpur University, Assam, India; SAIF, IIT Madras; STIC, Kochi University, Kochi and IIT, Kanpur. The authors are also grateful to UGC, New Delhi, India, for financial support under the SAP-DRS-I program (2016–2021).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baruah, J., Gogoi, R., Gogoi, N. et al. A thiosemicarbazone–palladium(II)–imidazole complex as an efficient pre-catalyst for Suzuki–Miyaura cross-coupling reactions at room temperature in aqueous media. Transit Met Chem 42, 683–692 (2017). https://doi.org/10.1007/s11243-017-0174-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0174-4