Abstract
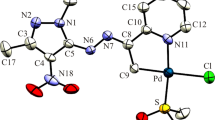
Complexes of the type cis-[PdX2(imzt)(PPh3)] {imzt = imidazolidine-2-thione; PPh3 = triphenylphosphine; X = Cl (1), Br (2), I (3), SCN (4)} have been synthesized and characterized by elemental analyses, molar conductance, IR and 1H NMR spectroscopies. The complex 1·MeOH was obtained from the reaction of [PdCl2(CH3CN)2], imidazolidine-2-thione and triphenylphosphine in CHCl3/CH3OH. Complexes 2·MeOH, 3 and 4 were prepared by metathesis of the chlorido ligands in 1 with bromide, iodide and thiocyanate, respectively. Elemental analyses showed good agreement with the expected mononuclear compositions, while the molar conductivities of the complexes in DMF were consistent with their nonelectrolytic nature. NMR spectra confirmed coordination of the imidazolidine-2-thione and triphenylphosphine ligands. Single-crystal X-ray diffraction determination of 1·CH3OH showed that the coordination geometry around PdII is nearly square planar, with the chlorido ligands in a cis configuration. All four complexes have been tested in vitro by XTT assay for their cytotoxicity against human glioblastoma cell line (U87MG). The binding of 1 with guanosine was studied by 1H NMR spectroscopy, revealing that the coordination takes place via N7.







Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) Acta Neuropathol 114(2):97–109
Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG (2006) Oncologist 11:165–180
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) N Engl J Med 352:987–996
Warnick RE, Prados MD, Mack EE, Chandler KL, Doz F, Rabbitt JE, Malec MK (1994) J Neurooncol 19(1):69–74
Roci E, Cakani B, Brace G, Bushati T, Rroji A (2014) Med Arch 68(2):140–143
Wang D, Lippard SJ (2005) Nat Rev Drug Discov 4(4):307–320
Medici S, Peana M, Nurchi VM, Lachowicz JI, Crisponi G, Zoroddu MA (2015) Coord Chem Rev 284(1):329–350
Garoufis A, Hadjikakou SK, Hadjiliadis N (2009) Coord Chem Rev 253:1384–1397
Ramos-Lima FJ, Quiroga AG, Garcıa-Serrelde B, Blanco F, Carnero A, Navarro-Ranninger C (2007) J Med Chem 50(9):2194–2199
Caires ACF, Almeida ET, Mauro AE, Hemerly JP, Valentini SR (1999) Quim Nova 22(3):329–334
Nadeem S, Bolte M, Ahmad S, Fazeelat T, Tirmizi SA, Rauf MK, Sattar SA, Siddiq S, Hameed A, Haider SZ (2010) Inorg Chim Acta 363:3261–3269
Rocha FV, Barra CV, Garrido SS, Manente FA, Carlos IZ, Ellena J, Fuentes ASC, Gautier A, Morel L, Mauro AE, Netto AVG (2016) J Inorg Biochem 159:165–168
Barra CV, Rocha FV, Morel L, Gautier A, Garrido SS, Mauro AE, Frem RCG, Netto AVG (2016) Inorg Chim Acta 446:54–60
Legendre AO, Mauro AE, Ferreira JG, Ananias SR, Santos RHA, Netto AVG (2007) Inorg Chem Commun 10:815–820
Netto AVG, Frem RCG, Mauro AE (2001) Mol Cryst Liq Cryst 374:255–260
Rocha FV, Barra CV, Mauro AE, Carlos IZ, Nauton L, Ghozzi ME, Gautier A, Morel L, Netto AVG (2013) Eur J Inorg Chem 25:4499–4505
Moro AC, Watanabe FW, Ananias SR, Mauro AE, Netto AVG, Lima APR, Ferreira JG, Santos RHA (2006) Inorg Chem Commun 9:493–496
Barra CV, Rocha FV, Gautier A, Morel L, Quilles MB, Carlos IZ, Treu-Filho O, Frem RCG, Mauro AE, Netto AVG (2013) Polyhedron 65:214–220
Moro AC, Cunha GA, Souza RFF, Mauro AE, Netto AVG, Carlos IZ, Resende FA, Varanda EA, Pavan FR, Leite CQF (2015) Med Chem Res 24:2879–2888
Sheldrick GM (2008) Acta Cryst A 68:112–122
Geary WJ (1971) Coord Chem Rev 7:81–122
Pearson RG (1973) Inorg Chem 12(3):712–713
Mak TW, Jasim KS, Chieh C (1984) Can J Chem 62:808
Raper ES, Creighton JR, Wilson JD, Clegg W, Milne A (1989) Inorg Chim Acta 155:85–89
Wheatley PJ (1953) Acta Cryst 6:369–377
Dwarakanath K, Sathyanarayana DN (1979) Bull Chem Soc Jpn 52:2699–2704
Deacon GB, Green JHS (1967) Spectrochim Acta A 24:845–852
Bowmaker GA, Chaichit N, Pakawatchai C, Skelton BW, White AH (2009) Can J Chem 87:161–170
Burmeister JL, Basolo F (1964) Inorg Chem 3:1587–1593
Ashraf W, Ahmad S, Isab AA (2004) Transit Metal Chem 29:400–404
Wazeer MIM, Isab AA (2007) Spectrochim Acta A 68:1207–1212
Montaldi AP, Sakamoto-Hojo ET (2013) Clin Exp Med 13:279–288
Barnham KJ, Bauer CJ, Djuran MI, Mazid MA, Rau T, Sadler PJ (1995) Inorg Chem 34:2826–2832
Chevry A, Teyssot ML, Maisonial A, Lemoine P, Viossat B, Traïkia M, Aitken DJ, Alves G, Morel L, Nauton L, Gautier A (2010) Eur J Inorg Chem 22:3513–3519
Gigli R, Pereira GJS, Antunes F, Bechara A, Garcia DM, Spindola DG, Jasiulionis MG, Caires ACF, Smaili SS, Bincoletto C (2016) Eur J Med Chem 107:245–254
Acknowledgements
This work was sponsored by Grants from FAPESP (proc. 2009/54011-8, 2012/15486-3 and 2016/17711-5), FAPEMIG, CNPq (proc. 475322/2009-6 and 422105/2016-3) and CAPES.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Moura, T.R., Cavalcanti, S.L., de Godoy, P.R.D.V. et al. Synthesis, characterization and antitumor activity of palladium(II) complexes of imidazolidine-2-thione. Transit Met Chem 42, 565–574 (2017). https://doi.org/10.1007/s11243-017-0161-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0161-9