Abstract
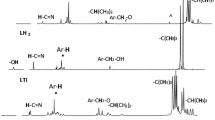
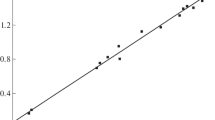
This paper describes the synthesis of (pyridyl)benzoazole Zn(II) and Cu(II) complexes and their applications as catalysts in ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). Reactions of 2-(3-pyridyl)-1H-benzimidazole (L1), 2-(2-pyridyl)-1H-benzothiazole (L2) and 2-(2-pyridyl)-1H-benzimidazole (L3) with Zn(II) and Cu(II) acetates produced the corresponding complexes; [Zn2(L1)2(OAc)4)] (1), [Cu2(L1)2(OAc)4] (2), [Zn(L2)(OAc)2)] (3), [Zn(L3)(OAc)2)] (4) and [Cu(L3), (OAc)2)] (5). Molecular structures of complexes 2 and 5a revealed that while L1 adopts a monodentate binding mode, through the pyridyl nitrogen atom, L3 exhibits a bidentate coordination mode. All the complexes formed active catalysts in the ROP of ε-CL to afford moderate molecular weight polymers. The kinetics of the ROP reactions of ε-CL were pseudo-first-order with respect to monomer and catalysts.







Similar content being viewed by others
References
Okada M (2002) Prog Polym Sci 27:87
Flieger M, Kantorova M, Prell A, Řezanka T, Votruba T (2003) Folia Microbiol 48:27
Wang Y, Zhao W, Liu D, Li S, Liu X, Cui D, Chen X (2012) Organometallics 31:4182
Wu JC, Yu TL, Chen CT, Lin CC (2006) Coord Chem Rev 250:602
Lindblad MS, Liu Y, Albertsson A-C, Ranucci E, Karlsson S (2002) Adv Polym Sci 157:139
Koeller S, Kadota J, Deffieux A, Peruch F, Massip S, Léger J-M, Desvergne J-P, Bibal B (2009) J Am Chem Soc 131:15088
Jensen TR, Schaller CP, Hillmyer MA, Tolman WB (2005) J Organomet Chem 690:5881
Zhong Z, Ankoné MJ, Dijkstra PJ, Birg C, Westerhausen M, Feijen J (2001) Polym Bull 46:51
Chuang HJ, Chen HL, Huang BH, Tsai TE, Huang PL, Liao TT, Lin CC (2013) J Polym Sci, Part A: Polym Chem 51:1185
Thomas CM (2010) Chem Soc Rev 39:165
Zoltowaska K, Sobczak M, Oledzka E (2015) Molecules 20:2816
Drouin F, Oguadinma PO, Whitehorne TJ, Prud’homme RE, Schaper F (2010) Organometallics 29:2139
Routary A, Nath N, Mantri S, Maharana T, Sutar AK (2015) Chin J Catal 36:764
Appavoo D, Omondi B, Guzei IA, Van Wyk JL, Zinyemba O, Darkwa J (2014) Polyhedron 69:55
Whitehorne TJ, Vabre B, Schaper F (2014) Dalton Trans 43:6339
Ojwach SO, Zaca TP (2015) S Afr J Chem 68:7
Attandoh NW, Ojwach SO, Munro OQ (2014) Eur J Inorg Chem, 3053
Ojwach SO, Okemwa TT, Attandoh NW, Omondi B (2013) Dalton Trans 42:10735
Beloglazkina E, Yudin I, Majouga A, Moiseeva A, Tursina A, Zyk A (2006) Russ Chem Bull 55:1803
Hein D, Alheim RJ, Leavitt J (1957) J Am Chem Soc 79:427
Bruker Group (2012) APEX2, SAINT and SADABS. Bruker AXS Inc., Madison
Sheldrick GM (2015) Acta Cryst 71:3–8
Farrugia LJ (2012) J Appl Crystallogr 45:849–854
Spek AL (2009) Acta Cryst D65:148–155
Motswainyana WM, Onani MO, Ojwach SO, Omondi B (2012) Inorg Chim Acta 391:93
Housecroft CE, Sharpe AG (2008) Inorganic Chemistry, 3rd edn. Pearson Education Ltd, Hallow, p 670
Li XP, Pan M, Zheng SR, Liu YR, He QT, Kang BS, Su CY (2007) Cryst Growth Des 7:2481
Jones CJ (2002) Tutorial Chemistry Texts, d and f block Chemistry, vol 4. Royal Society of Chemistry, Cambridge, p 73
Iqbal M, Ahmad I, Ali S, Muhammad N, Ahmed S, Sohail M (2013) Polyhedron 50:524
Casanova J, Alzuet G, Latorre J, Borrás J (1997) Inorg Chem 36:2052
Barret J (2003) Tutorial Chemistry Texts, Inorganic Chemistry In Aqueous Solution, vol 21. Royal Society of Chemistry, Cambridge, p 154
Sung CY, Li C-Y, Sun J-K, Chen TY, Lin C-H, Ko BT (2012) Dalton Trans 41:953
Romain C, Rosa V, Fliedel C, Bier F, Hild H, Welter R, Dagorne S, Avilés T (2012) Dalton Trans 41:3377
Vion JM, Jerome R, Teyssie P, Aubin M, Prudhomme RE (1986) Macromolecules 19:1828
Pilone Lamberti AM, Mazzeo M, Milione S, Pellecchia C (2013) Dalton Trans 42:13036
Acknowledgments
The authors would like to thank the University of KwaZulu-Natal for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11243_2016_66_MOESM1_ESM.docx
Supplementary Figures S1–S3 represent 1H NMR, ESI–MS spectra and crystal packing of complexes 4, 5 and 2, respectively. Figures S4 represents plots of ln[CL]0/[CL] t versus time versus time for catalysts 1–5, while Figure S5 depicts a plot of ln[CL]0/[CL] t versus time at different catalyst concentrations using 1. Typical GPC chromatogram of PCL sample is given in Figure S6, while ESI mass and 1H NMR spectra of the polymers are given in Figures S7 and S8. CCDC numbers 1404899 and 1404900 contain the supplementary crystallographic data for compounds 2 and 5a, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. (DOCX 1003 kb)
Rights and permissions
About this article
Cite this article
Zaca, T.P., Ojwach, S.O. & Akerman, M.P. Ring-opening polymerization of ε-caprolactone catalysed by (pyridyl)benzoazole Zn(II) and Cu(II) complexes. Transit Met Chem 41, 663–673 (2016). https://doi.org/10.1007/s11243-016-0066-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0066-z