Abstract
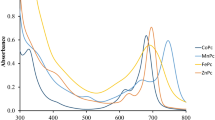
Phthalocyanine (Pc) compounds-containing α-naphtholbenzein units have been synthesized and characterized by spectroscopic data and elemental analysis. The redox properties of the compounds were investigated by cyclic and square wave voltammetry, controlled-potential coulometry and in situ spectroelectrochemistry in DMSO and compared with the free phthalonitrile ligand. The Pc compounds displayed common metal and/or ring-based reduction and oxidation processes. However, electrochemical measurements clearly suggested that the substituents involving anthraquinone units are redox active and so have a considerable effect on the redox processes of these compounds.








Similar content being viewed by others
References
Mc Keown NB (1998) Phthalocyanine materials-synthesis, structure and function. Cambridge University Press, New York
Krzystek J, Ozarowski A, Telser T (2006) Coord Chem Rev 250:2308–23024
Jiang JZ, Ng DKP (2009) Acc Chem Res 42:79–88
Altun S, Altındal A, Özkaya AR, Bulut M, Bekaroğlu Ö (2008) Tetrahedron Lett 49:4483–4486
Özer M, Altındal A, Özkaya AR, Bekaroğlu Ö (2009) Dalton Trans 17:3175–3181
Yılmaz F, Özer M, Kani İ, Bekaroğlu Ö (2009) Catal Lett 130:642–647
Yang F, Forrest SR (2008) ACS Nano 2:1022–1032
Eichorn H, Wöhrle D, Pressner D (1997) Liq Cryst 22:643–653
La Torre De G, Vazquez P, Agulla-Lopez F, Torres (1998) J Mater Chem 8:1671–1683
Abdurrahmanoğlu Ş, Özkaya AR, Bulut M, Bekaroğlu (2004) Dalton Trans 23:4022–4029
Rodriguez-Mendez ML, De Saja JA (2009) J Porphyr Phthalocya 13:606
Camerin M, Magaraggia M, Soncin M, Jori G et al (2010) Eur J Cancer 46:1910–1918
Ayhan MM, Durmuş M, Gürek A, Ahen V (2014) Synth Metals 195:83–90
Koçak M, Cihan A, Okur Aİ, Gül A, Bekaroğlu Ö (2000) Dyes Pigments 45:9–14
Sevim AM, Yenilmez HY, Aydemir M, Koca A, Bayır ZA (2014) Electrochim Acta 137:602–615
Orman EB, Koca A, Özkaya AR, Gürol İ, Durmuş M, Ahsen V (2014) J Electrochem Soc 161(6):422–429
Kılıçaslan MB, Kantekin H, Koca (2014) Dyes Pigments 103:95–105
Odabaş Z, Altındal A, Özkaya AR, Salih B, Bekaroğlu Ö (2010) Sens Actuators B: Chem 145:355–366
Ejele AE, Iwu IC, Enenebeaku C, Ukiwe LN, Okolue BN (2012) J Emerg Trends Eng Appl Sci 4:668–672
James AL, Chilvers KF, Perry JD, Armstrong L, Gould FK (2000) Appl Environ Microbiol 66:5521–5523
Özçeşmeci M (2014) J Org Chem 767:16–21
Perrin DD, Armarego WLF (1980) Purification of laboratory chemicals, 2nd edn. Pergamon, Oxford
Gaspard S, Mailard M (1987) Tetrahedron 43:1083–1090
Van Nostrum CF, Picken SJ, Schouten AJ, Nolte RJM (1994) Angew Chem Int Ed Engl 33:2173–2175
Turek P, Petit P, Andre JJ, Simon J et al (1987) J Am Chem Soc 109:5119–5122
Meller A, Ossko A (1972) Monatsh Chem 103:150–155
Çamur M, Özkaya AR, Bulut M (2007) Polyhedron 26:2638–2646
Odabaş Z, Koç İ, Altındal A, Özkaya AR, Salih B, Bekaroğlu Ö (2010) Synth Met 160:967–977
Sezer B, Şener MK, Koca A, Erdoğmuş A, Avcıata U (2010) Synth Met 160:2155–2166
Altun S, Özkaya AR, Bulut M (2012) Polyhedron 48:31–42
Lever ABP, Milaeva ER, Speier G, Leznoff CC, Lever ABP (eds) (1993) Phthalocyanines: properties and applications, vol 3. VCH, New York, p 5
Kissinger PT, Heineman WR (1996) Laboratory techniques in electroanalytical chemistry. CRC, New York and Basel
Koç İ, Çamur M, Bulut M, Özkaya AR (2009) Catal Lett 131:370–380
Başak AS, Özkaya AR, Altındal A, Salih B, Şengül A, Bekaroğlu Ö (2014) Dalton Trans 43:5858–5870
Salan Ü, Altındal A, Özkaya AR, Salih B, Bekaroğlu Ö (2012) Dalton Trans 41:5177–5187
Acknowledgments
This work was supported by the Scientific and Technological Research Council of Turkey (TUBİTAK) (Project No: 113Z860) and partly by The Turkish Academy of Sciences (TUBA).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sağlam, M.B., Özkaya, A.R. Electrochemical and in situ spectroelectrochemical properties of metal-free and metallophthalocyanines containing α-naphtholbenzein groups on the peripheral positions. Transit Met Chem 41, 605–612 (2016). https://doi.org/10.1007/s11243-016-0059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0059-y